To re-evaluate mitoxantrone for possible survival benefits in a larger contemporary sample and to demonstrate analytic uses of the newly available Project Data Sphere (PDS) online resource, data from control arms of completed clinical trials were used to compare survival and toxicity among patients with postdocetaxel metastatic castrate-resistant prostate cancer treated with mitoxantrone and prednisone. This study illustrates the potential for comparative effectiveness research studies using PDS.
Keywords: Prostate cancer, Comparative effectiveness research, Mitoxantrone, Postdocetaxel, Project Data Sphere
Abstract
Background.
Mitoxantrone was approved for use in metastatic castrate-resistant prostate cancer (mCRPC) based on pain palliation without observed survival benefit in a small phase III trial in 1996. To re-evaluate for possible survival benefits in a larger contemporary sample and to demonstrate analytic uses of the newly available Project Data Sphere online resource, we used data from control arms of completed clinical trials to compare survival and toxicity among patients with postdocetaxel mCRPC treated with mitoxantrone and prednisone.
Patients and Methods.
Control arm data from two phase III randomized control trials, SUN 1120 and TROPIC, were used to examine the efficacy of mitoxantrone plus prednisone (n = 305) versus prednisone alone (n = 257) among patients with postdocetaxel mCRPC. Propensity score matching was used to balance patient characteristics between the separate trials, conditioned on age and key prognostic variables of survival. The primary outcome was overall survival. Secondary endpoints evaluated safety.
Results.
Median survival was similar among patients receiving mitoxantrone plus prednisone versus prednisone alone (385 days vs. 336 days; deceleration factor = 0.04; 95% confidence interval: −0.12 to 0.22). Prevalence of several any-grade toxicity, including fatigue, back pain, and peripheral neuropathy, was increased among patients who received mitoxantrone.
Conclusion.
There was no significant survival benefit for mitoxantrone plus prednisone over prednisone alone among men with mCRPC after docetaxel therapy. This finding is consistent with prior studies showing no survival advantage with mitoxantrone in the predocetaxel setting. Furthermore, our data suggest that mitoxantrone may be associated with increased toxicity compared with prednisone alone.
Implications for Practice:
This study is the first to use clinical trial data from Project Data Sphere (PDS) to elucidate a current question in clinical oncology that is independent from the questions that the trials were originally designed to assess. This study illustrates the potential for comparative effectiveness research studies using PDS or similar repositories to answer questions for which prospective studies may never be conducted.
Introduction
Mitoxantrone was approved by the U.S. Food and Drug Administration (FDA) for use in metastatic castrate-resistant prostate cancer (mCRPC) in 1996 based on a palliative benefit with improvement in bone pain and effect on analgesic use [1–3]. Nearly two decades ago, in a small phase III trial, 161 patients with symptomatic mCRPC were randomized to mitoxantrone plus prednisone versus prednisone alone; pain relief response (the primary study outcome) was achieved in 29% of patients treated with mitoxantrone versus 12% of patients treated with prednisone, with no survival benefit observed [3]. A second study of 242 mCRPC patients randomized to mitoxantrone plus hydrocortisone versus hydrocortisone alone suggested improvement of pain severity and frequency with mitoxantrone but, again, was unable to demonstrate a survival benefit [1]. A third multicenter clinical trial comparing prednisone and mitoxantrone plus prednisone in 2002 demonstrated more frequent prostate-specific antigen (PSA) responses and longer time to treatment failure among 120 men with mCRPC, with no significant survival benefit for mitoxantrone [4].
Despite its purported palliative benefits, possible side effects of mitoxantrone include gastrointestinal distress; myelosuppression; fever; pain; and, less commonly, acute myelogenous leukemia and congestive heart failure [3]. Since the introduction of mitoxantrone in 1996, several therapeutic options including taxanes have become available for use in mCRPC. In the TAX 327 trial, docetaxel plus prednisone showed a significant survival benefit of approximately 3 months compared with mitoxantrone plus prednisone in mCRPC, leading to its FDA approval in 2004 [5]. Subsequently, cabazitaxel plus prednisone resulted in prolonged survival compared with mitoxantrone plus prednisone among patients with docetaxel failure in the TROPIC trial [6]. Given these advances, the current role and timing of mitoxantrone in the management of patients with mCRPC are unclear.
No available data satisfactorily answer the question of whether mitoxantrone offers any benefit, including survival, in patients with postdocetaxel mCRPC. A prospective randomized study is unlikely to be conducted, given the availability of newer agents (both approved and under study) and the lack of interest in investigating mitoxantrone in this setting. Nonetheless, mitoxantrone continues to be used in clinical practice among patients with progressive mCRPC and remains a required standard of care comparator for prospective studies investigating new agents in the post-taxane setting.
Comparative effectiveness research (CER) offers an opportunity to study the risks and benefits of competing standards of care outside the setting of prospective randomized studies. To date, most CER has been performed in large observational registry or claims-based data sets that provide very large numbers of exposed patients but may contain limited patient-level characteristics [7] In contrast, the Project Data Sphere (PDS) initiative represents a unique opportunity to perform CER analyses using clinical trial-grade data. Currently, comparator arm data from 14 clinical trials are available in the PDS database, and 12 involve prostate cancer. Available trial data can be pooled and analyzed using standard pharmacoepidemiologic methods for bias reduction, allowing for cross-trial comparisons of treatment effects.
To re-evaluate for possible survival benefits among patients with mCRPC in a larger contemporary sample and to demonstrate the potential analytic uses of the newly available PDS online resource, we used data from control arms of completed clinical trials in PDS to compare survival and toxicity between mitoxantrone plus prednisone and prednisone alone among patients with postdocetaxel mCRPC. We hypothesized that the combination of mitoxantrone plus prednisone would not be associated with a survival benefit when compared with prednisone alone in this mCRPC postdocetaxel population.
Patients and Methods
Study Overview and Design
In this retrospective cohort study, we reanalyzed data from the control arms of two phase III randomized controlled trials, SUN 1120 and TROPIC. SUN 1120 was initiated in 2008 to evaluate the safety and efficacy of sunitinib in combination with prednisone versus placebo and prednisone among 873 patients with mCRPC that progressed after treatment with docetaxel-based chemotherapy [8, 9]. The experimental arm (n = 584) received sunitinib 37.5 mg/day orally plus prednisone 5 mg twice daily, whereas the comparator arm (n = 289) received a placebo plus prednisone 5 mg twice daily. SUN 1120 was terminated prematurely on September 27, 2010, due to evidence that the combination of sunitinib plus prednisone was unlikely to improve overall survival compared with prednisone during a scheduled interim analysis.
The TROPIC trial, published in 2010, examined the safety and efficacy of cabazitaxel plus prednisone compared with mitoxantrone plus prednisone among 755 patients with mCRPC after disease progression with docetaxel-based chemotherapy [10]. All participants were treated with prednisone 10 mg daily. The experimental arm (n = 378) received cabazitaxel 25 mg/m2 intravenously over 1 hour every 3 weeks, whereas the comparator arm (n = 377) received mitoxantrone 12 mg/m2 intravenously over 15–30 minutes. Patients were treated until disease progression, death, unacceptable toxicity, or for a maximum of 10 cycles. For both trials, the primary outcome was overall survival. Inclusion and exclusion criteria for SUN 1120 and TROPIC are outlined in the supplemental online Appendix.
In the present study, control arm data from the SUN 1120 and TROPIC trials were used to examine the efficacy of mitoxantrone plus prednisone compared with prednisone alone among the patients with mCRPC refractory to docetaxel.
Study Population
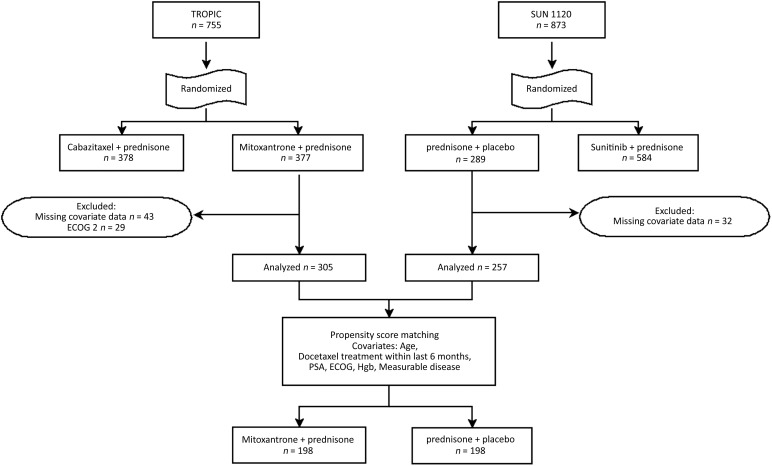
There were 289 patients in SUN 1120 and 377 patients in TROPIC who were potentially eligible for inclusion in this study. In order to ensure the comparability of the patients across these two trials, we limited our samples from each trial by excluding those who would not have been eligible for both trials. Specifically, we excluded patients with Eastern Cooperative Oncology Group (ECOG) scores of 2 because TROPIC allowed scores of 2 and SUN 1120 did not. Patients were also excluded if they were missing covariate information on any of the key variables for our analysis (Fig. 1). This resulted in 562 total patients, including 305 patients treated with mitoxantrone plus prednisone (TROPIC) and 257 patients treated with prednisone alone (SUN 1120), who were eligible for inclusion in our analysis.
Figure 1.
CONSORT diagram. Measurable disease if a lesion >10 mm was observed on spiral computed tomography (CT) or >20 mm was observed on conventional CT.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; Hgb, hemoglobin; PSA, prostate-specific antigen.
Measures
The primary outcome of interest was overall survival. Overall survival was considered from the date of randomization to the date of death from any cause, with a median follow-up time of 12.8 months in TROPIC and 8.7 months in SUN 1120.
For the outcomes of interest, we controlled for covariate data collected at baseline within each trial. Covariates included age and prognostic variables of survival in the postdocetaxel, second-line chemotherapy setting [11]. The prognostic variables included in our analysis included docetaxel treatment within the last 6 months, ECOG performance status (0 or 1), hemoglobin, serum PSA level, and measurable disease (presence of a lesion >10 mm on spiral computed tomography or >20 mm on conventional computed tomography).
The secondary endpoint was a comparison of safety data from each trial. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. AEs, physical exams, hematology, biochemistry, vital signs, and electrocardiograms were monitored throughout both studies. Left ventricular ejection fraction was monitored throughout the TROPIC trial in mitoxantrone-treated patients. For the purpose of this study, we focused on the most common AEs among any grade, grade 2, and grade ≥3.
Statistical Analysis
Propensity Score Estimation and Application
Because our study integrates data from two distinct trials, we lost the benefit of random assignment from the parent trials. To balance characteristics of patients from the separate trials, we used propensity score matching. We estimated the propensity score by modeling the probability of being in the prednisone arm versus the mitoxantrone plus prednisone arm, conditioned on the key covariates described above, using the R package MatchIt [12]. From 257 patients treated with prednisone and 305 patients treated with mitoxantrone plus prednisone, a comparable subset of 198 patients was selected from each study (match percentage of 76%). In evaluating the differences between patients who were matched and those who were not within each treatment group, we found that in both SUN 1120 and TROPIC, recent docetaxel, higher ECOG performance status, and higher hemoglobin were associated with being matched. Matched patients were older than unmatched patients in SUN 1120, whereas matched patients were younger than unmatched patients in TROPIC. These matched subsets were used in all subsequent analyses to assess the effects of mitoxantrone on survival of patients with progressive postdocetaxel mCRPC.
Survival Analysis
SAS version 9.3 (SAS Institute Inc., Cary, NC, http://www.sas.com) was used for all analyses. The Cox proportional hazards assumption was not met for all covariates included in our study, thus the survival analysis was performed using an accelerated failure time model with Weibull distribution. Deceleration factors and 95% confidence intervals (CIs) were calculated with an accelerated failure time model for the primary outcome of overall survival. Patients contributed time to the analysis until the date of death, disenrollment, or the end of the study. We had 80% power to detect a median survival difference of 25% between patients treated with mitoxantrone plus prednisone versus patients treated with prednisone alone (α = .05). In our secondary analysis of safety data, we evaluated the prevalence of adverse events occurring over a period of 28.7 months, the follow-up time from start to completion of the shorter trial in our analysis, SUN 1120.
Sensitivity Analysis
To ensure the robustness of our findings, we considered two alternative survival models. The first model used the propensity score-matched sample and did not adjust further for covariates, which were shown to be balanced, in our matched cohort. In the second model, we added each of the matching covariates to ensure that there was no residual confounding when using the propensity score matched sample. Results were consistent across these two models, thus we present the results from only the former model.
Results
Baseline Characteristics
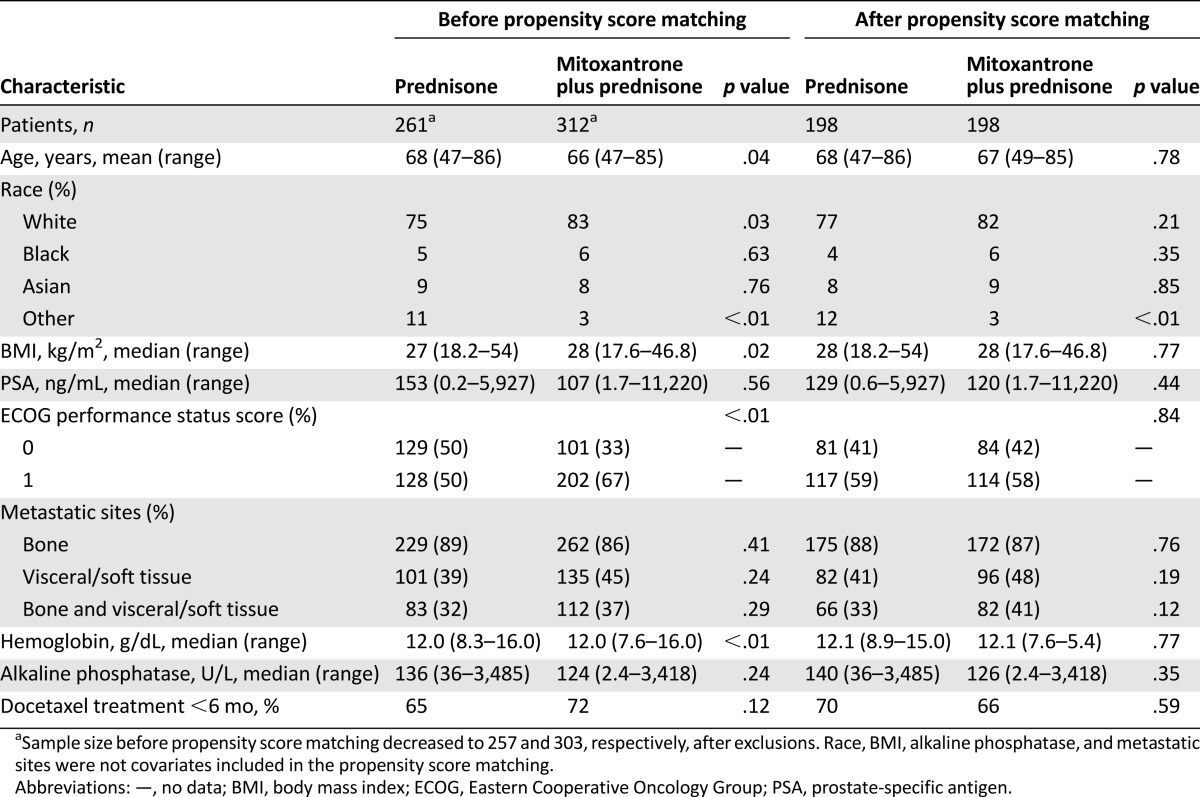
Prior to propensity score matching, patients in the two trials differed on several baseline characteristics (Table 1). On average, for example, patients in the mitoxantrone plus prednisone group were younger, had higher body mass index, were more likely to be white, and were less likely to include patients identified as “other” race compared with the prednisone group alone. Patients also differed in their baseline hemoglobin levels and ECOG performance status prior to matching. After matching, patients were similar to one another on all measured characteristics, with the exception of other race.
Table 1.
Comparison of characteristics of patients treated with mitoxantrone plus prednisone versus prednisone alone, before and after propensity score matching propensity score matching
Outcome
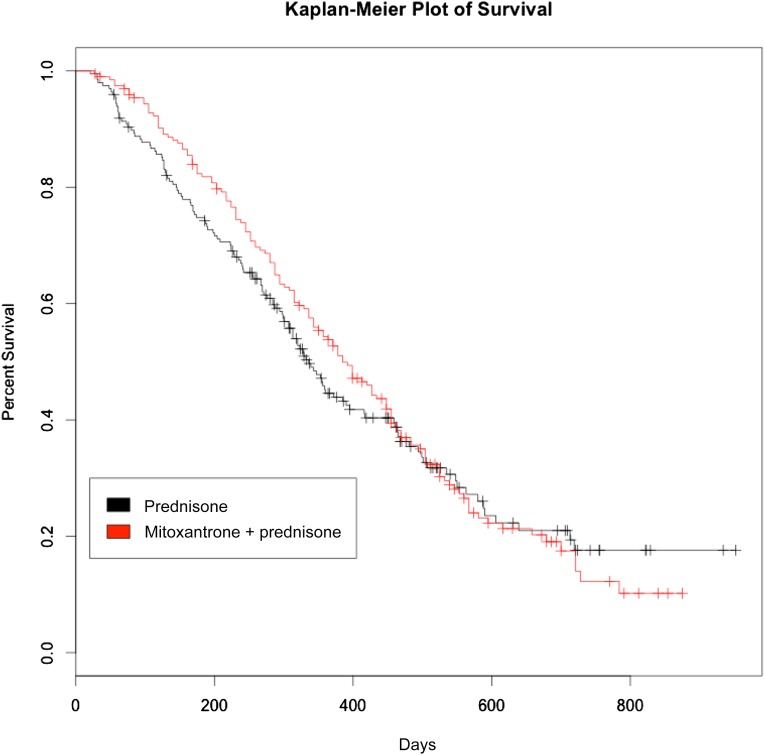
There was no significant difference in overall survival among those receiving mitoxantrone plus prednisone compared with those receiving prednisone alone in the accelerated failure time model. Median survival among patients who received mitoxantrone plus prednisone was 385 days compared with 336 days among patients who received prednisone alone (Fig. 2) (deceleration factor = 0.04; 95% CI: −0.12 to 0.22). Match status was not a significant predictor of overall survival in either treatment group. Adjustment for matching covariates in the model had no significant effect on the results.
Figure 2.
Kaplan-Meier estimates comparing overall survival between patients with docetaxel-refractory metastatic castrate-resistant prostate cancer treated with mitoxantrone plus prednisone versus prednisone alone.
Safety or Toxicity
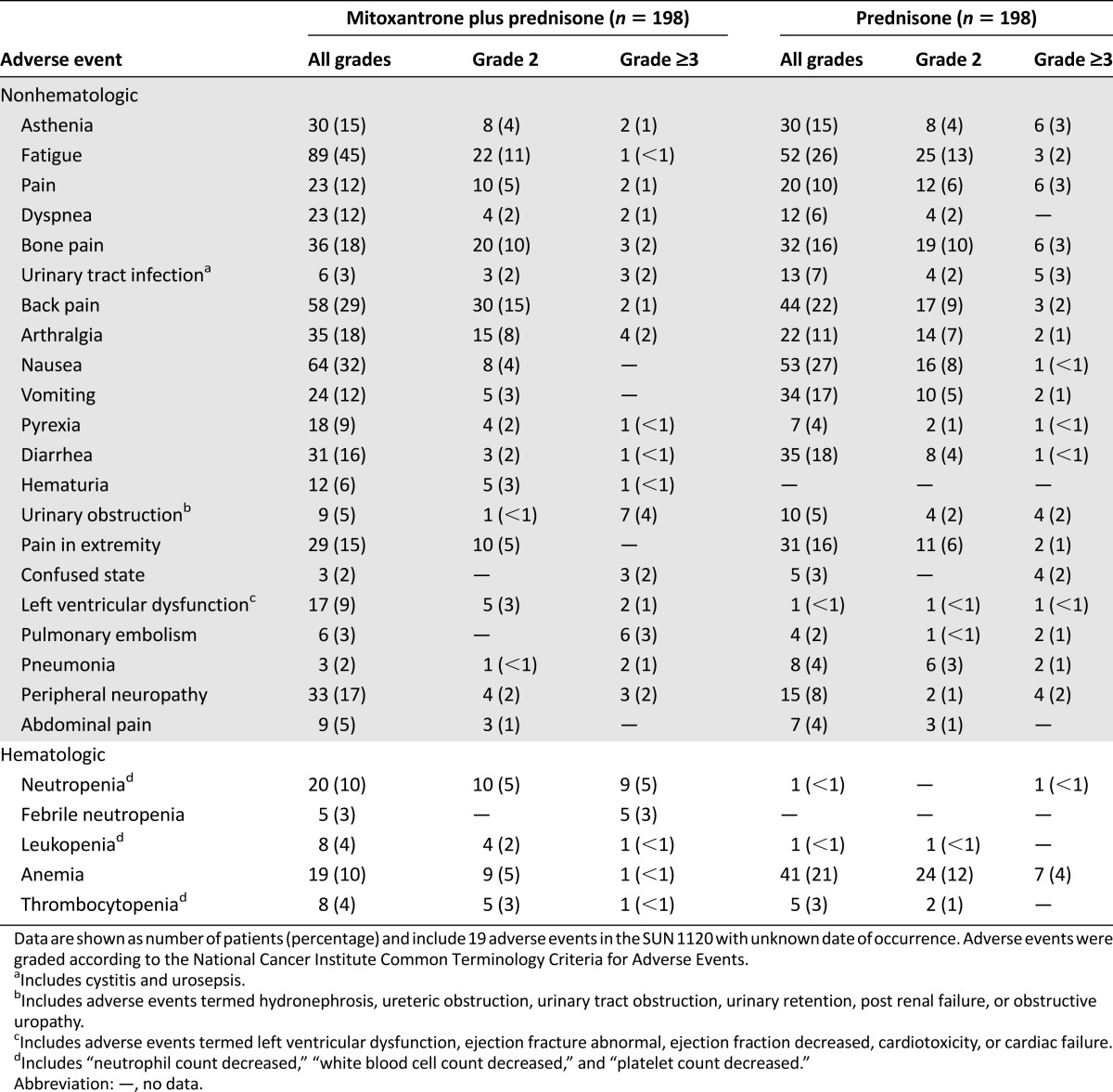
Common toxic effects of mitoxantrone plus prednisone versus prednisone alone are outlined in Table 2. A higher proportion of grade ≥3 neutropenia and neutropenic fever (3% vs. none) was reported among patients receiving mitoxantrone plus prednisone versus prednisone alone. A higher prevalence of anemia, among all grades, was noted among patients receiving prednisone alone, including 4% with grade ≥3 toxicity categorized as anemia. Among any-grade AE, fatigue (45% vs. 26%), peripheral neuropathy (17% vs. 8%), dyspnea (12% vs. 6%), and back pain (29% vs. 22%) were more prevalent among patients receiving mitoxantrone plus prednisone. Any grade of left ventricular dysfunction was also higher among those receiving mitoxantrone compared with patients receiving prednisone alone (9% vs. <1%). Both groups had <1% of patients with grade ≥3 toxicity related to left ventricular dysfunction.
Table 2.
Common hematologic and nonhematologic adverse events reported among patients who received mitoxantrone plus prednisone versus prednisone alone
Discussion
In this retrospective analysis comparing control arm data from two large, previously published randomized clinical trials, there was no significant survival benefit for mitoxantrone plus prednisone versus prednisone alone among men with mCRPC after docetaxel therapy. This finding is consistent with prior studies showing that mitoxantrone does not offer a survival advantage in the predocetaxel setting. Fatigue, peripheral neuropathy, dyspnea, and back pain were also more prevalent among patients receiving mitoxantrone plus prednisone. Grade ≥3 AEs were generally similar between the two study arms, with the exception of increased left ventricular dysfunction and neutropenic fever among patients receiving mitoxantrone and increased anemia among patients receiving prednisone alone. Signs of cardiotoxicity, however, may have been monitored more frequently among patients receiving mitoxantrone, given its known cardiac effects.
The current study has several important limitations. Although our data were derived from randomized clinical trials, our study population was not randomized between treatment and control arms. Consequently, similar to traditional observational studies, our analyses are subject to bias from confounding. To overcome this limitation, propensity score matching, based on previously described predictors of survival in mCRPC, was used to minimize this bias. We were unable to assess the influence of mitoxantrone on specific quality-of-life metrics because of a lack of comparable patient-reported symptoms or quality-of-life data from the two studies. Using available toxicity data, however, we demonstrated an increased prevalence of several important toxic effects among patients treated with mitoxantrone, raising questions about its previously asserted palliative benefits in the treatment of mCRPC. Our safety analyses were limited in that we did not have access to data distinguishing treatment-related AEs that led to dose reduction or delay in treatment. Furthermore, because of our small sample size (396 patients), power was insufficient to detect a significant difference in survival with mitoxantrone therapy. Finally, the SUN 1120 group received 5 mg of prednisone twice daily compared with 10 mg once daily in the TROPIC group, but this difference is likely clinically insignificant.
Our study is unique in that we coupled observational CER methodology with clinical trial data to answer a research question distinct from the primary objectives for either individual trial. By using data from comparator arms of two large phase III studies, we overcame many of the inherent limitations of standard retrospective comparative effectiveness studies, which often have limited clinical information regarding potentially important determinants of outcomes such as comorbidities, toxicities of treatment, and detailed data on treatment dose and schedule. Combining traditional epidemiologic methods with clinical trial data provides the ability to better control for confounding and other sources of potential bias because of the collection and availability of rich patient-level baseline covariates and outcomes while managing biases introduced from nonrandom comparisons through methods such as propensity score matching.
To our knowledge, this study is the first to be published using clinical trial data from the PDS online resource to elucidate a current question in clinical oncology. In our study, we found no significant survival benefit in the postdocetaxel setting by adding mitoxantrone to prednisone in comparison with prednisone alone. In addition, several important toxic effects were more prevalent among patients treated with mitoxantrone. This raises the question of whether mitoxantrone should be considered a standard of care in this setting for either routine clinical care or as a standard comparator arm in post-taxane clinical trials. Additional studies are warranted to further evaluate the palliative benefit of mitoxantrone in this setting.
Conclusion
Our study illustrates the potential for CER studies using PDS or similar repositories to answer questions for which prospective studies may never be conducted.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Footnotes
Editor's Note: See the related commentaries, “The Project Data Sphere Initiative: Accelerating Cancer Research by Sharing Data,” on page 464, and “The Prostate Cancer DREAM Challenge: A Community-Wide Effort to Use Open Clinical Trial Data for the Quantitative Prediction of Outcomes in Metastatic Prostate Cancer,” on page 459 of this issue.
Author Contributions
Conception/Design: Angela K. Green, Robert W. Corty, William A. Wood, Mathew Meeneghan, Katherine E. Reeder-Hayes, Ethan Basch, Matthew I. Milowsky, Stacie B. Dusetzina
Collection and/or assembly of data: Angela K. Green, Robert W. Corty, William A. Wood, Stacie B. Dusetzina
Data analysis and interpretation: Angela K. Green, Robert W. Corty, William A. Wood, Mathew Meeneghan, Stacie B. Dusetzina
Manuscript writing: Angela K. Green, William A. Wood, Mathew Meeneghan, Katherine E. Reeder-Hayes, Ethan Basch, Matthew I. Milowsky, Stacie B. Dusetzina
Final approval of manuscript: William A. Wood, Katherine E. Reeder-Hayes, Ethan Basch, Matthew I. Milowsky
Disclosures
The authors indicated no financial relationships.
References
- 1.Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: Results of the cancer and leukemia group B 9182 study. J Clin Oncol. 1999;17:2506–2513. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 2.Osoba D, Tannock IF, Ernst DS, et al. Health-related quality of life in men with metastatic prostate cancer treated with prednisone alone or mitoxantrone and prednisone. J Clin Oncol. 1999;17:1654–1663. doi: 10.1200/JCO.1999.17.6.1654. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: A Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 4.Berry W, Dakhil S, Modiano M, et al. Phase III study of mitoxantrone plus low dose prednisone versus low dose prednisone alone in patients with asymptomatic hormone refractory prostate cancer. J Urol. 2002;168:2439–2443. doi: 10.1016/S0022-5347(05)64163-8. [DOI] [PubMed] [Google Scholar]
- 5.Berthold DR, Pond GR, Roessner M, et al. Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: Relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clin Cancer Res. 2008;14:2763–2767. doi: 10.1158/1078-0432.CCR-07-0944. [DOI] [PubMed] [Google Scholar]
- 6.Bahl A, Oudard S, Tombal B, et al. Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the TROPIC trial. Ann Oncol. 2013;24:2402–2408. doi: 10.1093/annonc/mdt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter WR, Meyer AM, Abernethy AP, et al. A framework for understanding cancer comparative effectiveness research data needs. J Clin Epidemiol. 2012;65:1150–1158. doi: 10.1016/j.jclinepi.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunitinib plus prednisone in patients with metastatic castration-resistant prostate cancer after failure of docetaxel chemotherapy (SUN 1120). Available at http://clinicaltrials.gov/show/NCT00676650. Accessed April 8, 2015.
- 9.Michaelson MD, Oudard S, Ou Y-C, et al. Randomized, placebo-controlled, phase III trial of sunitinib plus prednisone versus prednisone alone in progressive, metastatic, castration-resistant prostate cancer. J Clin Oncol. 2014;32:76–82. doi: 10.1200/JCO.2012.48.5268. [DOI] [PubMed] [Google Scholar]
- 10.De Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 11.Halabi S, Lin CY, Small EJ, et al. Prognostic model predicting metastatic castration-resistant prostate cancer survival in men treated with second-line chemotherapy. J Natl Cancer Inst. 2013;105:1729–1737. doi: 10.1093/jnci/djt280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho DE, Imai K, King G, et al. MatchIt: Nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1–28. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.