Abstract
The neural mechanisms that underlie familiarity memory have been extensively investigated, but a consensus understanding remains elusive. Behavioral evidence suggests that familiarity sometimes shares sources with instances of implicit memory known as priming, in that the same increases in processing fluency that give rise to priming can engender familiarity. One underappreciated implication of this account is that patterns of neural activity that appear to index familiarity in a generic sense may instead reflect fluency-related precursors of recognition. In a novel illustration of this principle, we examined brain potentials during recognition tests for visual words. In two experiments, fluency was selectively enhanced for half of the test cues via masked repetition priming. Replicating previous findings, the proportion of words endorsed as “old” was greater for words immediately preceded by a matching masked word versus an unrelated one. In addition, N400 potentials were more positive for test cues preceded by matching versus unrelated masked words. Similar N400 differences were observed when false alarms were compared to correct rejections for the subset of unstudied words that were preceded by matching masked words. These N400 effects were topographically dissociable from other potentials that correlated with familiarity for studied words. We conclude that experiences of familiarity can have different neural correlates that signal the operation of distinct neurocognitive precursors of recognition judgments. Conceptualizations of the neural basis of recognition memory must account for a plurality of mechanisms that produce familiarity memory.
Keywords: Implicit memory, Explicit memory, Familiarity, Fluency, Priming, Masked priming, ERP
1. Introduction
Dual-process theories of recognition memory posit that recognition decisions can be supported by either familiarity or recollection (Aggleton & Brown, 2006; Eichenbaum, Yonelinas, & Ranganath, 2007; Mandler, 1980; Yonelinas, 2002). Familiarity refers to the impression that a stimulus has been previously encountered that is unsubstantiated by the retrieval of any relevant contextual details. For example, familiarity would support a conviction that a woman’s face had been encountered previously, even without any further recall. By contrast, recollection implies that contextual or other details regarding the prior event are also recalled, such as the woman’s name or the location of a prior encounter.
Extensive research efforts have recently been focused on understanding the neural processes that support recollection and familiarity. However, fundamental questions germane to this topic remain open. Whereas recollection is often believed to operate via a categorical or threshold process (e.g., Yonelinas, 1994; Yonelinas & Parks, 2007), most characterizations of familiarity posit a signal-detection process by which a global match is computed between a test cue and stored memory traces (Hintzman, 1988; Norman, 2010; Shiffrin & Steyvers, 1997). As such, patterns of neural activity that vary continuously with the strength of subjective familiarity experiences are often presumed to index this summation. However, it has been argued that recollection can also be graded or continuous, such that familiarity and weak recollection are difficult to dissociate (Slotnick, 2010; Wixted, 2007; Wixted, Mickes, & Squire, 2010). In addition, certain forms of implicit memory exhibit properties that are very similar to those of familiarity (for reviews, see Paller, Voss, & Boehm, 2007; Yonelinas, 2002). As a result, questions have been raised about the extent to which patterns of neural activity that have previously been attributed to familiarity in neuroimaging studies may instead reflect forms of implicit memory, such as enhanced fluency at conceptual or perceptual levels of processing (Voss & Paller, 2007; Voss, Hauner, & Paller, 2009; Voss, Lucas, & Paller, 2010a; Wang, Lazzara, Ranganath, Knight, & Yonelinas, 2010).
The last of these concerns relates to broader questions about the relationship between familiarity and priming, an expression of implicit or nonconscious memory observed in various types of specialized tests. Substantial evidence suggests that the same fluency signals that give rise to priming can sometimes guide conscious recognition memory (Cleary, 2004; Jacoby & Whitehouse, 1989; Parkin et al. 2001; Westerman, Lloyd, & Miller, 2002; Westerman, Miller, & Lloyd, 2003). For example, in a pioneering study, Jacoby and Whitehouse (1989) gave participants recognition memory tests for words. Unbeknownst to the participants, each test word was preceded by a 50-ms, masked presentation of a prime word that was either the same as the upcoming test word (here termed masked-prime same or MP-same trials), or a different word (here termed masked-prime different or MP-different trials). Although participants were unable to identify the prime words, the probability of a subsequent “old” decision was higher on MP-same relative to MP-different trials1. Moreover, findings from subsequent research suggest that this and similar fluency manipulations disproportionately influence familiarity as opposed to recollection (Miller, Lloyd, & Westerman, 2008; Rajaram & Geraci, 2000; Woollams, Taylor, Karayanidis, & Henson, 2008; but see Brown & Bodner, 2011; Kurilla & Westerman, 2008; Taylor & Henson, this issue). These and related findings support a fluency-attribution account of familiarity, according to which feelings of familiarity can reflect an unconscious inference about the source of fluent processing rather than a direct product of an underlying memory trace (Jacoby & Dallas, 1981).
One important but underappreciated implication of this theoretical account is that the neural correlates of familiarity are likely to differ according to the extent and types of fluency from which each instance of familiarity is derived. Indeed, priming is known to have multiple subtypes driven by dissociable forms of fluency, the most well-studied of which are conceptual and perceptual fluency (Henson, 2003; Schacter, Wig, & Stevens, 2007). In a recent review, Alter and Oppenheimer (2009) catalogued at least four additional subtypes of fluency for linguistic stimuli alone, including phonologic, lexical, syntactic, and orthographic fluency, and argued that manipulating fluency along any of these dimensions can produce essentially the same behavioral outcome within a given domain of judgment, including judgments of familiarity. It is thus perhaps surprising that familiarity tends to be discussed and operationalized as an amodal or unitary neural construct. Indeed, neuroimaging methods have typically been employed in search of generic familiarity markers, most often with the goal of establishing double dissociations between familiarity and recollection in order to provide evidence in favor of dual-process models of recognition. As such, steps are rarely taken to determine whether patterns of neural activity that covary with familiarity are more closely tied to one or more potential precursors of recognition.
Importantly, dual-process models may not be adequately captured by neural double-dissociations if familiarity has a variable relationship to multiple underlying memory signals. This notion may help to reconcile current controversies concerning putative neural correlates of familiarity. For instance, a popular but controversial position within the literature on event-related potentials (ERPs) has been that familiarity and recollection can be doubly dissociated through specific brain potentials known as FN400 and LPC, respectively. However, FN400 potentials are found in conjunction with familiarity for meaningful or verbalizable stimuli—such as words or nameable pictures—but generally not for nonverbal stimuli such as abstract patterns or nonsense words, even when these items evoke strong familiarity (Danker et al., 2008; Voss & Paller, 2007; Voss et al., 2010a). Several explanations have been proposed as to why the association between FN400 and familiarity breaks down in situations that are not amenable to conceptual stimulus processing. For example, some have suggested that conceptual processing simply engenders larger amounts of familiarity or increases reliance on familiarity relative to nonconceptual processing (e.g., Danker et al., 2008; Meyer, Mecklinger, & Friederici, 2007). Others have proposed that FN400 potentials reflect conceptual fluency that occurs incidentally during recognition tests, and that LPC potentials reflect both recollection and familiarity per se (e.g., Voss & Paller, 2007; Voss et al., 2010a). Interestingly, fluency-attribution accounts of familiarity suggest a different hypothesis that has received little attention, which is that FN400 reflects a conceptual fluency-related precursor to familiarity. In other words, FN400 effects may often—but not always— correlate with familiarity because familiarity is often—but not always—derived from conceptual fluency. In addition to reconciling the aforementioned familiarity literature, this account can accommodate findings that FN400 potentials correlate with conceptual priming (Voss & Paller, 2006; Voss, Schendan, & Paller, 2010b).
It is difficult to probe neural correlates of conceptual fluency in isolation from familiarity because the conditions most suitable for producing conceptual fluency—such as repetition following deep or meaning-based encoding—often also produce familiarity. Thus, findings that similar ERPs are elicited during tests of conceptual priming and tests of familiarity could indicate a shared fluency source, but could also reflect contamination by one form of memory during tests intended to capture the other. Fluency-attribution accounts predict that whenever any fluency—including perceptual or lexical fluency—is attributed to prior exposure, its neural measures will be coupled with the resulting feeling of familiarity. As previously mentioned, these forms of fluency can be reliably achieved using masked priming manipulations, which can also provide behavioral evidence of the influence of this fluency on recognition decisions. The present research thus seeks further evidence to adjudicate on these issues by examining electrophysiological correlates of familiarity in situations wherein its source can be convincingly tied to fluency induced by masked-priming methods.
Our research strategy extends that used by Woollams et al. (2008), in which masked repetition priming of recognition test words was combined with EEG recordings. By analyzing ERPs, Woollams and colleagues were able to compare neural correlates of masked priming with those of familiarity for previously studied words. As predicted, masked priming was associated with increased familiarity (as assessed in a Remember/Know paradigm, a method for measuring recollection and familiarity via metacognitive judgments, Rajaram, 1993). Also, a comparison of familiar hits with misses, collapsed across MP-same and MP-different trials, revealed the expected FN400 effect. Although masked priming served to increase familiarity, it did not influence FN400 potentials, as would be expected if FN400 were a generic or universal index of familiarity. Rather, MP-same trials were associated with central ERPs from 150–250 ms as well as with posterior N400 potentials.
These findings support the idea that familiarity can be multiply determined, in that multiple neural signals were associated with familiarity. However, there were limitations of the extent to which ERPs associated with masked priming could be linked to the influence of masked priming on recognition. Indeed, these ERPs did not interact with behavioral indices of recognition memory, but rather were similar across recollection hits, familiarity hits, and correct rejections. These ERPs may thus have reflected fluency signals incidental to familiarity. The present research builds on these findings by interrogating relationships between neural correlates of masked priming and familiarity measures. Specifically, we reasoned that a suitable way to examine the relationship between masked priming and familiarity would be to compare ERPs to false alarms versus correct rejections. For MP-same items in particular, this comparison would be sensitive to the added fluency that biases a new item to be endorsed as old (false alarm) versus correctly endorsed as new (correct rejection). Due to the low false-alarm rate obtained by Woollams et al. (2008), such a comparison was not feasible. As previously mentioned, a parallel comparison was made for old words; however, those words could also have been familiar due to retrieval of study-phase information, which could have obscured relationships between masked priming and familiarity. Thus, the present research includes a first study (Experiment 1) to replicate the key findings from Woollams and colleagues, and a second study (Experiment 2) using a paradigm that was modified to obtain a larger number of false alarms.
2. Experiment 1: Methods
2.1. Participants
Twenty healthy adults between 18 and 23 years of age (mean = 20.6 years, SE = 0.34, 15 female, 17 right-handed) participated in the experiment and received monetary compensation. Data from an additional seven participants were collected but excluded due to excessive electroocular or muscle artifacts (> 25% of trials).
2.2. Materials
Stimuli consisted of 480 words, each 4–7 letters in length, which were selected from the Medical Research Council database described by Coltheart (1981). The old/new status and MP-same/MP-different status of the word sets were counterbalanced across participants. An additional 60 words were used in filler trials, as described below. All words were presented in black against a white background. A black fixation dot appeared in the center of the screen during each interstimulus interval (ISI).
2.3. Procedure
The experiment consisted of four study-test blocks. In each study phase, 60 words were presented in a random order bounded by filler words (two primacy buffers and two recency buffers). In each test phase, participants completed a recognition test in which the 60 old words from the previous study phase were intermixed with 60 new words. Half of the trials in each test phase were MP-same (i.e., an old or new word that was preceded by a masked presentation of the same word), and the remaining were MP-different (i.e., an old or new word that was preceded by a masked presentation of a different word that occurred in the same block). Masks took the form of non-alphanumeric character strings, each nine characters in length. One forward mask and two backward masks sandwiched each prime word in the test phase. To maintain consistency between study and test phases, forward- and backward-masked letter strings were interspersed with study words during each study phase. All study and test words were presented in upper case, and all masked words were presented in lower case. In studies that use masked priming paradigms with word stimuli, primes and targets are typically presented in different letter cases to help to ensure that observed effects reflect lexical rather than purely visual processing. Here, we followed this convention primarily for the sake of consistency with this literature and particularly with the procedures used by Woollams et al. (2008). Participants were not informed about the presence of the masked words. Rather, they were told only that flickering character strings would be interspersed with both study and test words, and that these “flickers” would be used by the experimenter to obtain a baseline measure of brain activity2.
Each study trial began with a fixation dot for 200 ms, followed by a 35-ms forward mask, a 35-ms presentation of a nonword (a randomly generated string of 4–7 letters), and then two consecutive 35-ms backward masks. The fixation dot was then shown again for 495 ms, followed by a 306-ms study word and then a 1694-ms fixation dot. Participants were instructed to indicate using button presses whether they found each word to be relatively interesting (Button 1) or relatively uninteresting (Button 2), using the index and middle finger of the dominant hand, respectively. Participants were also told to try to remember the words for the upcoming memory test.
Each test phase was preceded by two practice trials (with one new and one old filler word, data not included in analyses). Each test trial began with the message “Press Button 6 for the next trial.” After a 918-ms delay following the participant’s key press, a 35-ms forward mask was presented, followed by a 35-ms matching or non-matching prime word, and then two consecutive 35-ms backward masks. The fixation dot was then shown again for 495 ms, followed by a 306-ms test word and then a fixation dot that appeared until the participant’s response. Participants were instructed to maintain fixation during the test trials and to avoid blinking as much as possible. A longer interval was allowed between the masked word and the test word than had been used in the prior study (Woollams et al., 2008) on the assumption that providing time for sufficient processing of the meaning of the test word could allow consequences of that processing to become apparent earlier in the ERP to the test word (although this extra time did not seem to influence the ERP findings appreciably). Participants were instructed to indicate using button presses whether they thought each word was old (Button 1) or new (Button 2). Both speed and accuracy were emphasized. If the participant pressed Button 2, the next trial began. If the participant pressed Button 1, the prompt “Remember or Know?” appeared on the screen, informing participants to press Button 1 if they experienced recollection or Button 2 if they experienced familiarity. Recollection was defined as the retrieval of one or more contextual details from the study phase accompanying recognition of a stimulus. Recollection was explained prior to beginning the experiment using examples of possible details, such as recalling whether a word was labeled as interesting or uninteresting during the study phase, recalling a thought or feeling that the word evoked, or any other relevant detail. Familiarity was defined as a belief that the word was previously encountered without any accompanying contextual details. Participants received practice distinguishing between recollection and familiarity in a short practice block that contained 5 study and 10 test trials prior to beginning the experiment.
To encourage participants to pay attention at the time the masked primes appeared, there were also a small number of “catch” trials in which flicker stimuli were omitted, such that the test cue appeared at the time the forward mask would usually appear. Each study phase included 5 catch trials and each test phase included 10 catch trials. Catch trials utilized filler words and were not included in analyses.
Event-related potentials were extracted from scalp electroencephalographic recordings from 21 tin electrodes embedded in an elastic cap. Electrode locations adhered to the 10–20 system. Voltage was referenced to a right mastoid electrode and rereferenced offline to averaged mastoids. The electrooculogram was recorded from four additional channels using electrodes below the center of each eye and on each outer canthus. Electrode impedance was below 5 kΩ. Signals were recorded with a band pass of 0.05–200 Hz, and sampled at a rate of 1000 Hz (Neuroscan synamps). Each 1100-ms averaging epoch began 200 ms prior to stimulus onset. Mean prestimulus amplitudes were subtracted to correct for baseline variability. Epochs containing electroocular or other artifacts were excluded from ERP analyses (mean = 12.7%, SE = 0.01). Statistical comparisons were performed using repeated-measures ANOVA (criterion p = 0.05) with Greenhouse–Geisser correction for non-sphericity where appropriate.
3. Experiment 1: Results and discussion
3.1. Behavior
The mean percentages of responses in each condition are depicted in Table 1. Participants indicated that a word was “old” (i.e., that it appeared in the study phase) either with a recollection response (“Remember” or R) or with a familiarity response (“Know” or K). Correct recognition thus included two trial types for old words, R hits and K hits. New-word trials were either false alarms (FA) or correct rejections (CR). Overall accuracy, computed as Pr(Hit–FA), was 0.40 for R judgments and 0.18 for K judgments. For both types of recognition response, accuracy was reliably greater than 0 [t(19) = 11.26, p < .001 for R judgments; t(19) = 4.27, p < .001 for K judgments], indicating that memory was above chance levels.
Table 1.
Mean percentage of responses in each condition in Experiment 1. SE in parentheses.
| Remember | Know | New | |||||
|---|---|---|---|---|---|---|---|
| Studied | MP-same | 41.9 | (4.1) | 32.3 | (2.8) | 25.8 | (3.6) |
| MP-different | 40.6 | (4.0) | 32.4 | (2.7) | 27.0 | (3.7) | |
| Unstudied | MP-same | 2.2 | (1.0) | 16.5 | (2.7) | 81.3 | (3.3) |
| MP-different | 1.3 | (1.0) | 13.3 | (2.5) | 85.5 | (2.9) | |
Masked priming influenced recognition judgments in the expected manner, in that MP-same trials elicited a greater percentage of old judgments than did MP-different trials. To formally assess masked priming, a 2 (study status: studied/unstudied) × 2 (response type: R/K) × 2 (masked priming: MP-same/MP-different) ANOVA was performed on the percentage of old responses. The masked priming effect was confirmed by a significant main effect [F(1,19) = 5.2, p = .034]. The only interaction that approached significance was a trend for an interaction between study status and masked priming [F(1,19) = 3.2, p = .091], as the masked priming effect tended to be greater for unstudied than for studied words3.
The main effect of masked priming was significant for unstudied words analyzed separately [F(1,19) = 5.67, p = 0.03], but not for studied words analyzed separately [F(1,19) = 1.25; p = .28]. In neither case was there a significant interaction between response type and masked priming [F(1,19) = 1.58, p = .22 for unstudied words; F(1,19) = 0.34, p = .57 for studied words].
In summary, we replicated past findings in obtaining a higher proportion of “old” responses on MP-same trials than on MP-different trials, particularly with false alarms. Unlike in most previous research, this increase was not selective to “Know” responses. Because this null interaction is atypical, we conducted exploratory analyses to further probe effects of masked priming on “Remember” and “Know” responses. Separate 2 (study status: studied/unstudied) × 2 (masked priming: MP-same/MP-different) ANOVAs were performed for R and K responses. No main effect of masked priming emerged for R responses in isolation [F(1,19) = 2.0, p = .17] or for K responses in isolation [F(1,19) = 1.8, p = .20]. The study status × masked priming interaction was also nonsignificant for R responses [F(1,19) = 0.61, p = .81]. However, this interaction was marginal for K responses [F(1,19) = 3.49, p = .08], indicating that the increase in “old” responses for MP-same relative to MP-different K false alarms was greater than was the corresponding increase for K Hits. Additional paired t-tests revealed a marginal difference between MP-same and MP-different false alarms [t(19) = 1.87, p = .08], whereas the corresponding difference for hits was nonsignificant [t(19) = .07, p = .94]. In short, the primary analyses demonstrated masked priming effects across all trial types (hits and false alarms, R and K responses), while posthoc analyses revealed trends for effects of masked priming to be strongest for “Know” false alarms.
3.2. ERPs—Basic memory effects
ERPs from the test phase were first analyzed without considering masked priming in order to compare ERPs for R hits, K hits, and misses. Specifically, we examined patterns of neural activity that co-varied with familiarity by contrasting K hits with misses, and that co-varied with recollection by contrasting R hits with K hits.
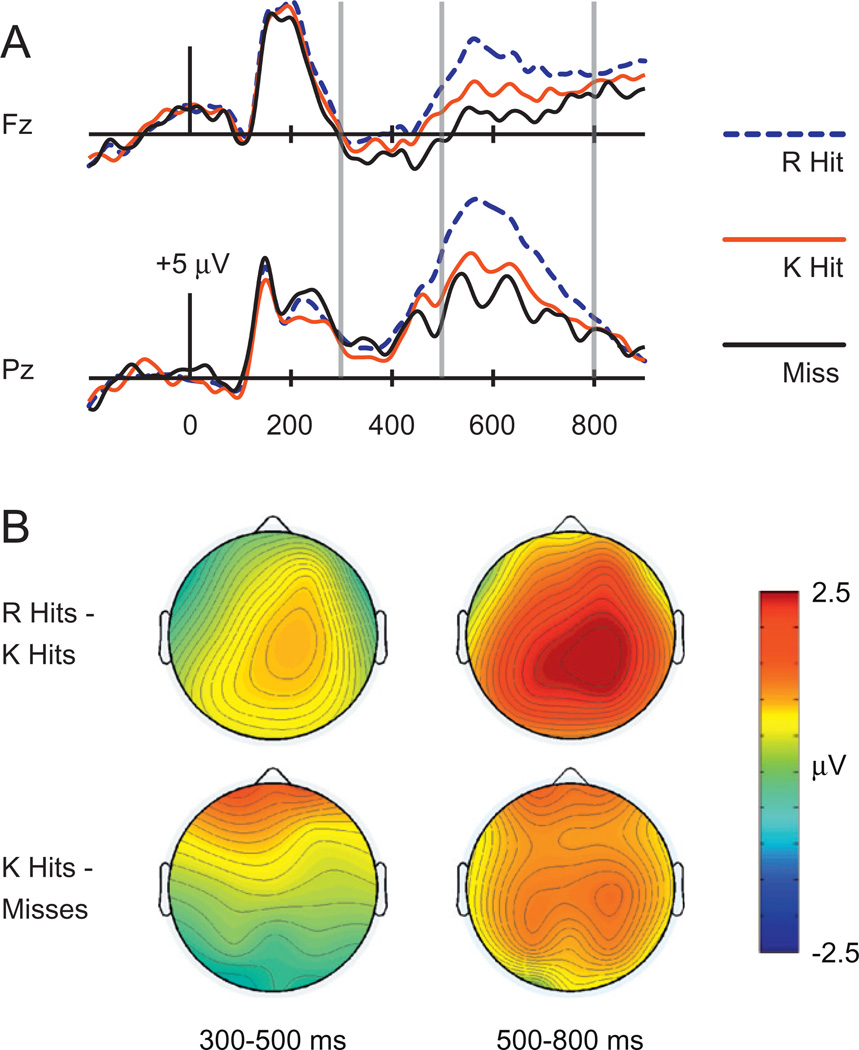
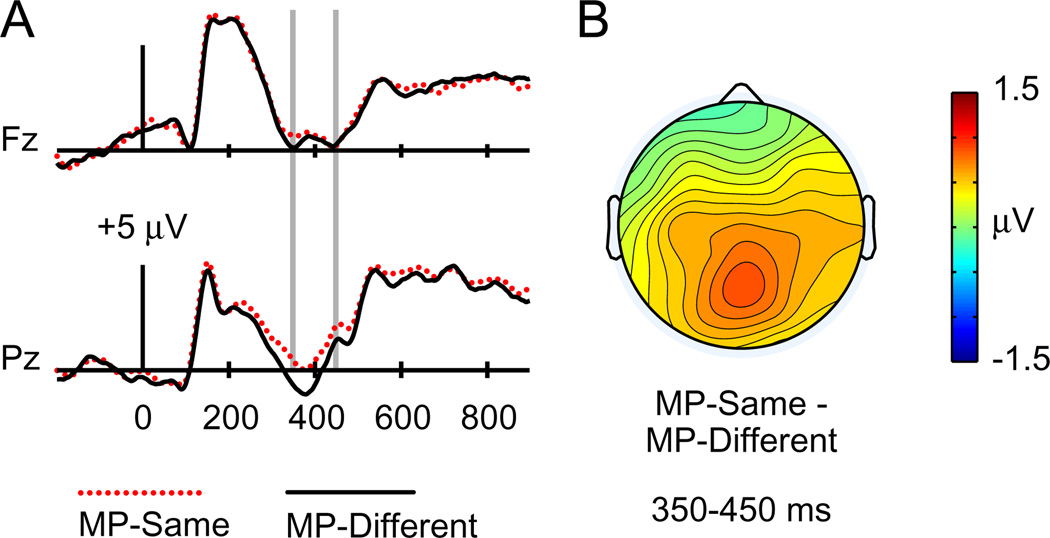
Fig. 1 shows that at approximately 300 ms after test word onset, positive hit/miss effects with maximum values at frontal electrodes were visible for old words endorsed with recollection and familiarity relative to old words that were missed. These frontal differences encompassed the 300–500 ms range typically ascribed to FN400 effects, and continued through the end of the epoch. In addition, later positive effects with posterior distributions (500–800 ms, LPC effects) were evident for old words endorsed with recollection relative to those endorsed with familiarity or misses.
Fig. 1.
ERPs related to recollection (“Remember” hits or R hits), familiarity (“Know” hits or K hits), and misses, collapsed across masked priming conditions in Experiment 1. (A) Waveforms for each condition are shown for midline frontal electrode Fz and midline parietal electrode Pz. Gray vertical lines indicate time windows of interest (300–500 ms and 500–700 ms). (B) Topographical plots depict ERP differences between R hits and K hits (top) and between K hits and Misses (bottom).
Formal ERP comparisons across these three conditions over the 300–500 and 500–800 ms latency intervals were performed at frontal electrode Fz and posterior electrode Pz. A 3 × 2 × 2 ANOVA was conducted with factors response type (R-hit/K-hit/miss), latency (300–500 ms/500–800 ms), and electrode (Fz/Pz). A three-way interaction indicated that ERP differences between response types differed across space and time [F(1.72,32.58) = 5.25, p = .01]. Assessments were thus made separately for each electrode and interval.
For the 300–500 ms interval, the main effect of response type was significant at both Fz [F(1.77,33.54) = 9.1, p = .001] and Pz [F(1.52,28.88) = 4.15, p = .04]. Follow-up paired t-tests revealed significantly more positive amplitudes for K hits relative to misses at Fz [t(19) = 2.49, p = .02] but not at Pz [t(19) = 0.10, p = .93]. By contrast, amplitudes at Pz were significantly more positive for R hits than for K hits [t(19) = 3.73, p = .001]. This difference was marginal at Fz [t(19) = 2.02, p = .06]. There were significantly more positive amplitudes for R hits relative to misses at both Fz [t(19) = 3.77, p = .001] and Pz [t(19) = 2.31, p = .03].
For the interval from 500–800 ms, the main effect of response type was also significant at both Fz [F(1.42,26.96) = 19.66, p < .001] and Pz [F(1.51,28.68) = 23.8, p < .001]. Follow-up paired t-tests revealed significantly more positive amplitudes for R hits relative to K hits at Fz [t(19) = 4.65, p < .001] and Pz [t(19) = 7.01, p < .001]. Likewise, amplitudes were significantly more positive for R hits relative to misses at Fz [t(19) = 4.87, p < .001] and Pz [t(19) = 5.68, p < .001]. Amplitudes were significantly more positive for K hits than misses at Fz [t(19) = 2.97, p = .008] and marginally so for K hits than misses at Pz [t(19) = 1.89, p = .07].
In sum, consistent with the prior literature on ERP effects during recognition memory tasks, these analyses revealed a difference between K hits and misses that was greater at frontal than at posterior electrodes, and a difference between R hits and K hits that was greatest at posterior electrodes. Formal assessments of topographic distributions of the aforementioned ERP effects utilized the vector-normalization approach (McCarthy & Wood, 1985). Averaged amplitude values from each electrode were compared for two conditions after overall amplitude differences were removed. This comparison sought to determine if the topography of the difference between K hits and misses from 300 to 500 ms differed reliably from the difference between R hits and K hits from 500 to 800 ms. A significant electrode-by-condition interaction [F(4.49,85.24) = 8.91, p < .001] substantiated the observation of a more anterior effect in the former compared to the latter contrast.
3.3. ERPs—Masked priming effects
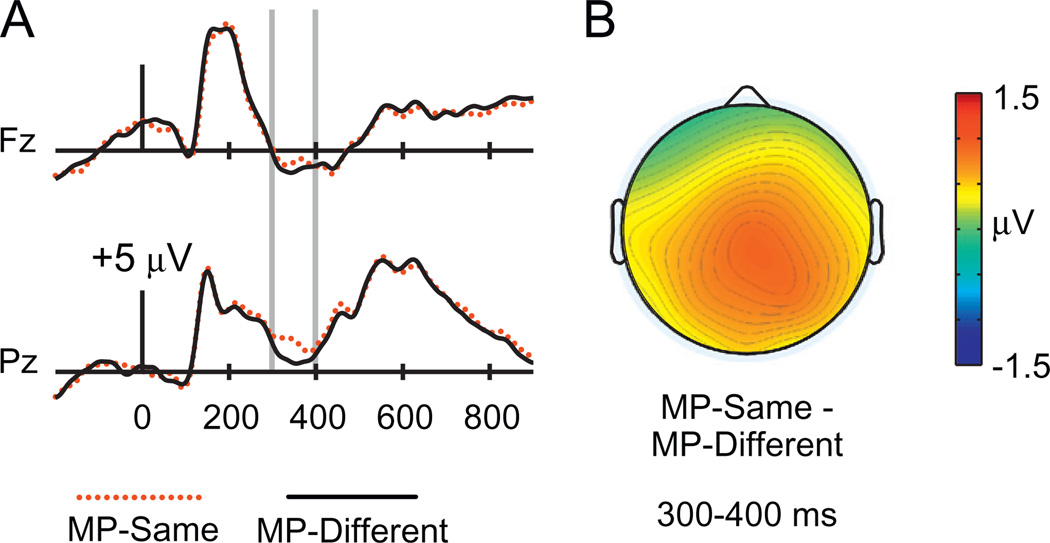
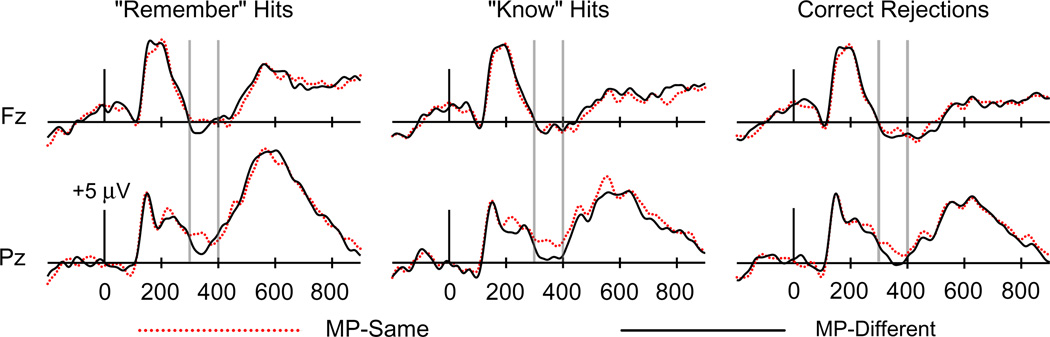
To examine masked priming effects, we first collapsed across response type and old/new status to examine overall differences between MP-same and MP-different trials. As shown in Fig. 2, amplitudes from 300 to 400 ms were more positive for MP-same relative to MP-different trials, consistent with the typical latency of N400. This difference was most pronounced at posterior electrodes. Formal analysis at electrode Pz confirmed significantly more positive amplitudes for MP-same relative to MP-different test cues [F(1,19) = 9.81, p = .005]. No difference was present at electrode Fz [F(1,19) = 1.61, p = .22]. This parietal effect was similar across the three response types (Fig. 3), as confirmed using a 2 × 3 ANOVA on ERPs at electrode Pz with factors masked priming (MP-same/MP-different) and response type (R hit/K hit/CR), which revealed a nonsignificant interaction [F(1.95,36.99) = .62, p = .54]. Formal analyses of topographic distribution confirmed that the topography of this masked priming effect differed reliably from that of the frontal FN400 effect identified for K hits versus misses [F(2.77,52.57) = 6.08, p = .002].
Fig. 2.
ERPs to test words preceded by matching masked primes (MP-same test words) and nonmatching masked primes (MP-different test words) in Experiment 1. (A) Waveforms are shown from midline frontal electrode Fz and midline parietal electrode Pz. Gray vertical lines indicate the time window of interest (300–400 ms). (B) A topographical plot depicts ERP differences between MP-same and MP-different test words.
Fig. 3.
ERPs for “Remember” hits, “Know” hits, and correct rejection trials as a function of MP-same versus MP-different status in Experiment 1. Waveforms are shown from midline frontal electrode Fz and midline parietal electrode Pz. Gray vertical lines indicate the time window of interest (300–400 ms).
Woollams et al. (2008) found an earlier, centrally-focused masked priming effect from 150 to 250 ms. No differences at this latency were present here, as indicated by a nonsignificant effect of masked priming when assessed from 150 to 250 ms at electrode Cz [F(1,19) = .097, p = .76].
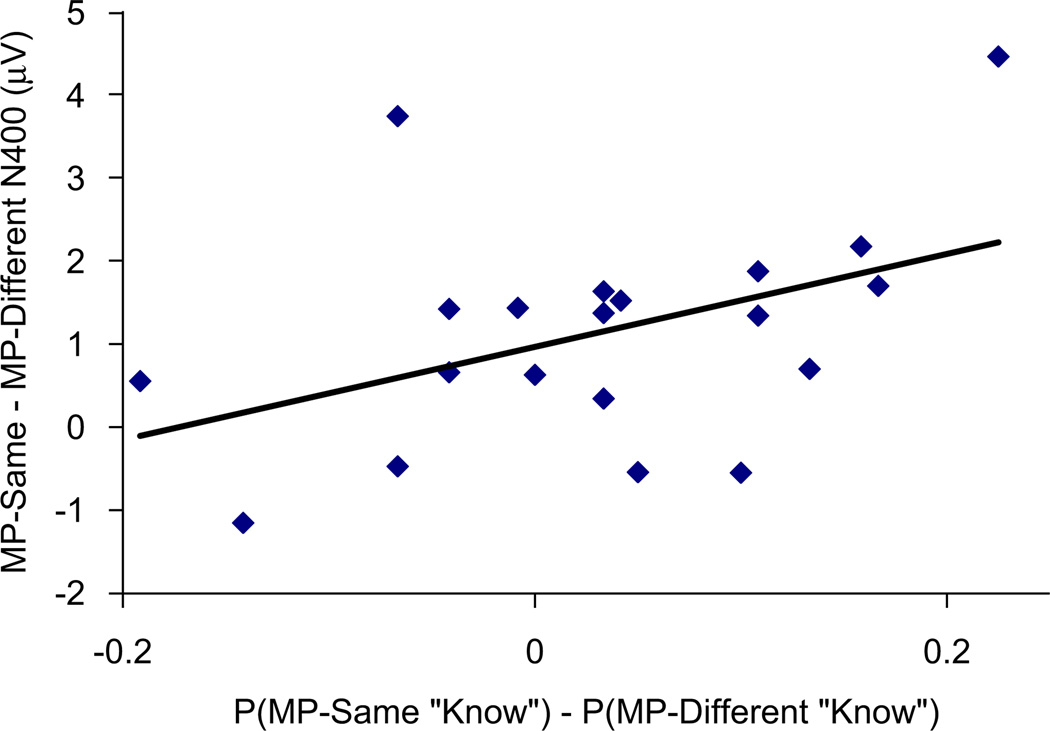
Relationships between N400 correlates of masked priming and corresponding behavioral effects were substantiated by additional across-participant correlational analyses. Effects of masked priming on “Know” responses were calculated as: (MP-same “Know” hits+MP-same “Know” false alarms)-(MP-different “Know” hits+MP-different “Know” false alarms). A similar index of masked priming effects on “Remember” responses was calculated for each subject. Masked priming effects on ERPs were quantified as the difference between MP-same trials and MP-different trials at electrode Pz from 300 to 400 ms. A marginal correlation was obtained between MP-related ERP differences and corresponding increases in “Know” responses [r(18) = .42, p = .065, Fig. 4]. No relationship was found between these ERP measures and increases in “Remember” responses [r(18) = −.07, p = .77]. Thus, participants who showed larger differences in posterior ERPs between MP-same and MP-different trials also showed greater increases in “Know” responses for MP-same compared to MP-different trials.
Fig. 4.
Across-subject correlation between the effects of masked priming on “Know” Responses and reduction in ERPs from 300–400 ms at Pz for MP-same relative to MP-different test words in Experiment 1.
4. Experiment 1: Discussion
In addition to replicating the behavioral Jacoby-Whitehouse masked priming effect, we replicated two key ERP findings from Woollams et al. (2008). First, MP-same words were associated with more positive posterior N400 potentials relative to MP-different words. Second, these N400 effects were topographically distinct from frontal potentials found in the contrast between K hits and misses. Thus, electrophysiological correlates of masked priming differed from those of familiarity-based recognition.
Some findings from Woollams et al. (2008) were not replicated in our study. Specifically, only in the earlier study was masked priming associated with an enhanced positivity at central electrodes from 150 to 250 ms. Though we cannot conclusively explain this divergence, it might be attributable to our use of a somewhat longer masked prime-target SOA (635 ms in our study versus 47 ms in the study by Woollams and colleagues). Pre-N400 effects are more commonly found with SOAs of less than 200 ms, possibly indicating greater fluency enhancement on pre-lexical levels of processing (Holcomb & Grainger, 2006, 2007). Our findings are thus consistent with this prior literature, and also suggest that masked priming effects on familiarity judgments in Woollams et al. (2008) were not driven entirely via pre-lexical levels. In the present experiment, N400 effects due to masked-priming showed a marginal correlation across subjects with increases in familiarity due to priming, providing tentative evidence for a connection between these fluency-related ERPs and familiarity.
A more puzzling difference between our results and those of Woollams et al. (2008) is the absence of differential effects of masked priming on reports of familiarity versus recollection. Unlike many previous studies using similar masked priming manipulations (Miller et al., 2008; Westerman, 2008; Westerman et al., 2002, 2003; Woollams et al., 2008), the effect of masked priming on recognition memory here was not selective to “K” responses. It is difficult to provide an explanation for this discrepancy, particularly given that our instructions were closely aligned with those of Woollams et al., 2008 and other prior studies. A potential clue as to why masked priming might sometimes have affected “R” responses came from anecdotal reports of a few participants who, during debriefing, mentioned occasionally having had the odd experience of realizing that they “had just been thinking about” a word that appeared on the screen during a memory test, seemingly by coincidence. Thus, it is possible that participants may sometimes have made the (incorrect) inference that thoughts related to an MP-same word were on their minds because they were recollecting these thoughts from the prior study phase. Although speculative, this explanation resonates with suggestions put forth by Andrew Mayes and colleagues that all mnemonic experiences, including experiences of recollection, can result partially from the attribution of fluency to prior experience (e.g., Mayes, Gooding, & van Eijk, 1997; Mayes & Roberts, 2001; see also Jacoby, Kelley, & Dywan, 1989; Kurilla & Westerman, 2008). Here, masked primes that were processed sufficiently so as to bring relevant thoughts to mind may have also triggered information typically associated with the prime word, thus increasing the fluency of associations between the word and other information. It is this type of associative fluency that Mayes et al. (1997) suggested to partially underlie experiences of recollection (see also Taylor and Henson, this issue, for evidence relevant to these issues).
Of course, it is important to keep in mind that the absence of interactions between masked priming and R/K responses in these data may simply have been due to insufficient statistical power. Indeed, exploratory analyses of masked priming effects limited to “Remember” and “Know” responses revealed that masked priming was not statistically robust for either response type in isolation, although there was a marginal effect of masked priming on “Know” responses when false alarms were considered in isolation. Moreover, it is unlikely that the masked-priming-related N400 modulations that we observed were linked solely to any influence of masked priming on recollection for at least two reasons. First, the marginal correlation between these ERP effects and increases in “Know” responses for MP-same words did not extend to corresponding increases in “Remember” responses. Second, similar N400 effects were observed in association with masked repetition priming in Woollams et al. (2008), even though the behavioral effects of masked priming were selective to “Know” responses. Whether and when the fluency signals that can be experienced as familiarity also give rise to recollection remains an open question. In Experiment 2, however, we attempt to circumvent this issue by focusing specifically on the effects of masked priming on false alarms. Because false alarms are overwhelmingly associated with familiarity instead of recollection (i.e., < 2% of unstudied trials were given “R” responses in Experiment 1), concentrating on false alarms mitigates the need to collect introspective reports of recollection and familiarity. Instead, Experiment 2 relies on old/new decisions in conjunction with confidence ratings.
As previously argued, focusing on masked-priming effects for false alarms is advantageous because, on these trials, retrieval of study-phase information has less of an influence on brain activity. Indeed, in Experiment 1—as in the study by Woollams et al. (2008)—masked priming effects on N400 potentials were similar across participants’ response types (R hits, K hits, and correct rejections). Thus, links between these masked-priming-related ERPs and different types of recognition experience were relatively indirect. We reasoned that, because analyses of masked priming effects on ERPs for recollection and familiarity concerned trials with recognized old words, retrieval of study-phase information was likely to have predominated ERP responses, perhaps with interactive processing of retrieved information and information from masked primes. Accordingly, relationships between masked priming effects and familiarity experiences may have been obscured by other ERPs that reflect other aspects of retrieval. Experiment 2 addressed this shortcoming using a modified paradigm in order to increase the proportion of false alarms. Specifically, we doubled the ratio of new to old words in the test phase, but informed participants of an equal ratio. Providing misinformation to participants that overstates the proportion of studied items on a recognition test has previously been found to encourage a liberal response criterion and to enhance fluency-based responding (e.g., Verfaellie, Giovanello, & Keane, 2001; Verfaellie & Cermak, 1999; Westerman et al., 2002). Thus, we reasoned that we could increase the probability of finding fluency-driven false alarms using this manipulation. In addition, we weakened explicit memory for studied words by speeding up the study phase and employing a shallow encoding task.
5. Experiment 2: Methods
5.1. Participants
Twenty-four healthy adults between 18 and 35 years of age (mean = 21 years, SE = .82, 19 female, 23 right-handed) participated in the experiment and received monetary compensation. Data from an additional six participants were collected but excluded due to excessive electroocular or muscle artifacts (n = 4, >25% of trials), failure to complete the experiment (n = 1) or for registering fewer than 15 false alarms for MP-same unstudied test cues (n = 1).
5.2. Materials
Stimuli were the same as those in Experiment 1. As in Experiment 1, the old/new status and MP-same/MP-different status of the word sets were counterbalanced across participants.
5.3. Procedure
Experiment 2 consisted of four study-test blocks. In each study phase, 40 words were presented in a random order in either a red or a green font. Font color was randomly assigned to each word. In each test phase, participants completed a recognition test in which the 40 old words from the previous study phase were intermixed with 80 new words. All test words appeared in a black font. Participants were misinformed that the ratio of old to new words was 1:1. As in Experiment 1, half of the trials were MP-same and half MP-different. All study and test words were presented in uppercase. All masked words were presented in lowercase. Participants were not informed about the presence of the masked words.
Each study trial began with a fixation dot for 200 ms, followed by a 23-ms presentation of a forward mask, a randomly-generated string of 4–7 letters presented for 35 ms, and then two consecutive 35-ms backward masks. The fixation dot was then shown again for 495 ms, followed by a 153-ms presentation of the study word. Participants were instructed to indicate using button presses whether each word was presented in a red font (Button 1) or a green font (Button 2), using the index and middle finger of the dominant hand, respectively. Participants were also told to try to remember the words for the upcoming memory test, though they were instructed that completing the font color task should take priority over attempting to remember the words.
As in Experiment 1, each test phase was preceded by two practice trials, which were excluded from analysis. Each test trial began with the message “Press Button 6 for the next trial.” After a 918-ms delay following the participant’s key press, a 23-ms forward mask was presented, followed by a 35-ms matching or non-matching prime word, and then two consecutive 35-ms backward masks. The fixation dot was then shown again for 495 ms, followed by a 306-ms test word and then a fixation dot that appeared until the participant’s response. Unlike in Experiment 1, participants in Experiment 2 were not prompted to make “Remember/Know” decisions for test trials that were assigned an “old” response. Instead, they were instructed to indicate using a single button press for each item whether they felt confident that the word was old (Button 1), believed that the word was old but without confidence (Button 2), believed that the word was new without confidence (Button 3) or were confident that the word was new (Button 4). Buttons 1–4 corresponded to the first four fingers of the dominant hand.
Event-related potentials were extracted from scalp electroencephalographic recordings from 32 Ag/AgCl electrodes (BioSemi ActiveTwo system) at locations from the 10–20 system. Voltage was rereferenced offline to averaged mastoids. The electrooculogram was recorded from four additional channels using electrodes below the center of each eye and on each outer canthus. Signals were recorded with a band pass of 0–104 Hz, and sampled at a rate of 512 Hz. Signals were high-pass filtered offline at .05 Hz. Each 1100-ms averaging epoch began 200 ms prior to stimulus onset. Mean prestimulus amplitudes were subtracted to correct for baseline variability. Epochs containing electroocular or other artifacts were excluded from ERP analyses (mean = 12.6% SE = 0.01). Statistical comparisons were performed using repeated-measures ANOVA (criterion p = 0.05) with Greenhouse-Geisser correction for non-sphericity where appropriate.
6. Experiment 2: Results
6.1. Behavior
The mean percentages of responses in each condition are depicted in Table 2. As expected, masked priming influenced recognition judgments such that MP-same trials elicited a greater percentage of old judgments than did MP-different trials. To formally assess the effects of masked priming on recognition, a 2 (study status: studied/unstudied) × 2 (masked priming: MP-same/MP-different) × 2 (confidence: high-confidence/low-confidence) ANOVA was performed on the percentage of old judgments. This analysis revealed a significant main effect of masked priming [F(1,23) = 14.63, p = .001], and a marginal interaction between confidence and masked priming [F(1,23) = 4.04, p = .056]. These effects reflected a greater percentage of “old” judgments for MP-same than for MP-different items, and a trend toward a greater effect of masked priming on high-confidence responses4. The masked priming × study status interaction was not significant [F(1,23) = 0.93, p = .35], nor was the masked priming × study status × confidence interaction [F(1,23) = 0.40, p = .53].
Table 2.
Mean percentage of responses in each condition in Experiment 2. SE in parentheses.
| “Old” high confidence |
“Old” low confidence |
“New” low confidence |
“New” high confidence |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Studied | MP-same | 34.9 | (3.2) | 30.7 | (2.0) | 27.1 | (2.2) | 7.3 | (1.1) |
| MP-different | 31.5 | (2.8) | 29.8 | (1.7) | 30.5 | (2.1) | 8.2 | (1.1) | |
| Unstudied | MP-same | 16.3 | (2.3) | 26.0 | (1.5) | 41.3 | (2.5) | 16.4 | (2.0) |
| MP-different | 11.4 | (1.5) | 25.0 | (1.6) | 42.8 | (2.1) | 20.9 | (2.4) | |
Because electrophysiological comparisons in Experiment 2 focus on unstudied items, we analyzed masked priming effects on recognition behavior separately for unstudied items. A 2 (masked priming: MP-same/MP-different) × 2 (confidence: high-confidence/low-confidence) ANOVA was performed on the percentage of “old” judgments that were registered for new items (e.g., the percentage of false alarms). A main effect of masked priming was present [F(1,23) = 9.53, p = .005], reflecting a greater proportion of false alarms for MP-same relative to MP-different items. In addition, a significant interaction between masked priming and confidence emerged [F(1,23) = 7.63, p = .01]. This interaction reflected a stronger effect of masked repetition priming on high-confidence relative to low-confidence false alarms. Paired comparisons indicated a significantly greater proportion of high-confidence false alarms for MP-same relative to MP-different items [t(23) = 3.93, p = .001]. The analogous comparison for low-confidence false alarms was nonsignificant [t(23) = 0.92, p = .37].
6.2. ERPs—Masked priming and false recognition
The goal of Experiment 2 was to use trials with unstudied words to isolate the neural correlates of familiarity induced by masked repetition priming. The advantage of emphasizing unstudied items is that ERPs related to masked-priming-induced familiarity can be examined while eliminating the potentially confounding influence of prior study-phase exposure. All analyses are collapsed across confidence levels due to low trial counts (< 15) for high-confidence false alarms registered in many of the participants (n = 9 for MP-same high-confidence false alarms and n = 12 for MP-different high-confidence false alarms).
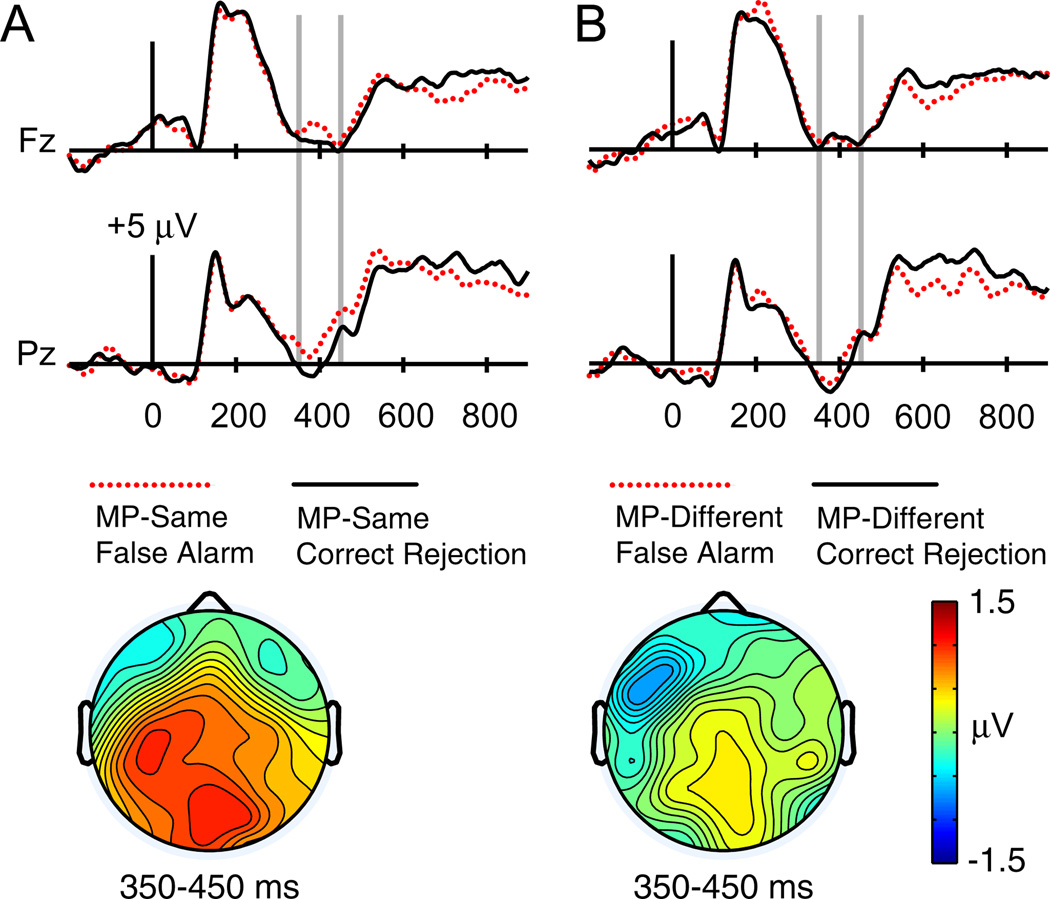
As shown in Fig. 5, visual inspection of the grand average waveforms for all participants revealed more positive amplitudes from 350 to 450 ms—consistent with the latency of N400—for MP-same relative to MP-different unstudied words. This difference was most pronounced at posterior electrodes. Similar ERP differences were found when participants’ responses were taken into account (Fig. 6). Specifically, a comparison between false alarms and correct rejections, collapsed across masked priming, revealed similar posterior N400 differences. A 2 × 2 masked priming (MP-same/MP-different) × response (FA/CR) ANOVA was thus conducted on mean amplitudes from 350 to 450 ms at Pz. This comparison yielded significant main effects of both masked priming [F(1,23) = 7.64, p = .01] and response [F(1,23) = 6.05, p = .02]. Thus, N400 potentials here were more positive both for MP-same relative to MP-different unstudied items, and for unstudied items that yielded false alarms relative to those that yielded correct rejections. The masked priming x response interaction was not significant [F(1,23) = 0.50, p = .49]5. Planned comparisons between false alarms and correct rejections conducted separately for MP-same and MP-different items revealed a significant difference for MP-same false alarms relative to MP-same correct rejections6 [F(1,23) = 4.58, p = .043] but not for MP-different false alarms relative to MP-different correct rejections [F(1,23) = 0.88, p = .36].
Fig. 5.
ERPs to unstudied test words preceded by matching masked primes (MP-same) and to unstudied test words preceded by nonmatching masked primes (MP-different) in Experiment 2. (A) Waveforms are shown from midline frontal electrode Fz and midline parietal electrode Pz. Gray vertical lines indicate the time window of interest (350–450 ms). (B) A topographical plot depicts ERP differences between MP-same and MP-different unstudied test words.
Fig. 6.
ERPs for false alarm and correct rejection trials as a function of MP-same versus MP-different status in Experiment 2. (A) ERPs to unstudied test words preceded by matching masked primes that were rated as “old” (MP-same false alarms) and those that were rated as “new” (MP-same correct rejections) in Experiment 2. (B) ERPs to unstudied test words preceded by non-matching masked primes that were rated as “old” (MP-different false alarms) and those that were rated as “new” (MP-different correct rejections). Waveforms are shown from midline frontal electrode Fz and midline parietal electrode Pz. Gray vertical lines indicate the time window of interest (350–450 ms).
A similar 2 × 2, masked priming × response ANOVA conducted at electrode Fz revealed no main effect of masked priming [F(1,23) = 0.59, p = .45], main effect of response [F(1,23) = 0.57, p = .46], or masked priming × response interaction [F(1,23) = 1.08, p = .31]. Focused comparisons between false alarms and correct rejections subdivided between MP-same and MP-different items at this electrode likewise revealed no significant differences [F(1,23) = 1.55, p = .23 for MP-same, F(1,23) = 0.07, p = .79 for MP-different].
7. Experiment 2: Discussion
In Experiment 1, ERP comparisons related to familiarity induced by masked priming could not be examined in isolation from ERPs related to familiarity induced by study-phase exposure. Because only studied words that were recognized with familiarity could be examined, all MP-same trials that were endorsed as familiar could have been familiar partially or entirely as a result of study-phase exposure rather than as a result of the masked priming manipulation. Design modifications in Experiment 2 allowed our analyses to focus on unstudied words, and N400 potentials were found to differ not only according to masked prime type (same or different word), but also according to the extent to which MP-same items were experienced as familiar (producing a false alarm as opposed to a correct rejection). Specifically, more positive N400 amplitudes were elicited by MP-same false alarms relative to MP-same correct rejections. Thus, N400 ERPs served as an electrophysiological index of both fluency and familiarity under these circumstances.
Another intriguing result from Experiment 2 was the preferential effect of masked priming on high-confidence responses. Although we had no strong a priori predictions regarding confidence in this experiment, the finding that fluency affected high-confidence responses contradicts the results of a previous study (Tunney & Fernie, 2007) in which a similar masked priming paradigm was used in the context of a recognition test. In this prior study, participants were given the option to “guess” that an item was old in addition to the options of responding with “remember,” “know,” or “new.” Masked priming increased only the proportion of “guess” responses. This pattern is provocative because it raises the possibility that fluency effects on recognition memory may not always reflect the attribution of fluency to familiarity, as is commonly assumed. Rather, fluency may guide participants’ behavior in a manner that is entirely unaccompanied by the subjective feeling of prior exposure that is characteristic of familiarity—a phenomenon termed recognition without awareness or implicit recognition (e.g., Voss & Paller, 2009a, 2010; Voss, Baym, & Paller, 2008). However, the present findings suggest that—at least under some circumstances—the influence of fluency on recognitions decisions is yoked to subjective experiences of recognition. Several differences between the present study and that of Tunney and Fernie (2007) might have affected the relationship between fluency and confidence levels. For example, overall recognition performance was higher in Tunney and Fernie’s study than it was in Experiment 2. Perhaps confidence judgments occur on a relative scale, such that the presence of very strong memories (i.e., those accompanied by recollection) can reduce the rated confidence associated with the use of fluency. The relationship between fluency-driven recognition and subjective memory experiences is an important topic for further investigation (see Voss, Lucas, & Paller, 2012, for a review of other factors that may influence this relationship). With respect to these data, however, an attributional model by which fluency served as a precursor to recognition experiences provides a better account than does an explanation based on recognition without awareness.
It is important to note that we cannot rule out the possibility that some proportion of the trials that attracted false alarms were associated with feelings of recollection in addition to feelings of familiarity. As previously noted, experiences of recollection for unstudied items are rare, and prior findings suggest that the link between N400 potentials and masked-priming induced fluency holds even when this fluency selectively affects familiarity-based responding (Woollams et al., 2008). However, the lack of selectivity of masked priming to “Know” responses in Experiment 1, combined with the finding that the effect of masked priming on false alarms in Experiment 2 was greatest for high-confidence responses, leaves open the possibility that participants may have experienced some amount of illusory recollection as a result of the masked priming manipulation. A useful question for future research will thus be to determine whether the ERPs that are associated specifically with fluency-induced recollective experiences differ from those associated with familiarity, or whether the N400 potentials observed here can serve as a precursor to either type of mnemonic experience.
8. General discussion
The present research was motivated by a disconnect between long-standing fluency-attribution accounts of familiarity—which posit that familiarity memory can be driven by multiple forms of fluency—and the homogenous manner in which familiarity is often characterized in relevant neuroimaging experiments. Neuroimaging investigations often rely on an “exclusion” method of operationalizing familiarity, according to which any behavioral or neural index of recognition that is not accompanied by recollection is attributed to familiarity. When familiarity is defined in this manner, its observed neural correlates could actually reflect forms of fluency that co-occur with or contribute to familiarity in some circumstances but are not universally related to familiarity. Thus, care must be taken to avoid misidentifying neural correlates of fluency as generic markers of familiarity. Instead, experimental methods must be applied to help define relationships that obtain between fluency and familiarity.
To this end, we experimentally manipulated the fluency of recognition test cues and observed relationships between electrophysiological correlates of masked priming-induced fluency and familiarity. Using the procedures for masked repetition priming during an explicit recognition test (as first introduced by Jacoby and Whitehouse, 1989) we computed several ERP contrasts in which we compared familiarity induced by this fluency manipulation to familiarity induced by prior study-phase exposure. As in prior ERP studies, when familiarity was examined for studied words (e.g., by comparing “know hits” to “misses”, Experiment 1), familiarity was indexed by differences in frontal potentials beginning around 300 ms, consistent with patterns of activity ascribed to FN400 potentials. Moreover, these ERPs were topographically distinct from posterior N400 correlates of masked priming. Importantly, when masked primes were used to induce false recognition for words that were not previously studied (Experiment 2), N400 potentials tracked not only the presence or absence of matching masked primes, but also the extent to which words were experienced as familiar. Specifically, N400 potentials (but not FN400 potentials) differentiated between MP-same false alarms and MP-same correct rejections. Thus, N400 differences appeared to signal a contribution of masked-priming-induced fluency to familiarity.
As previously stated, prior research on masked priming techniques using words suggests that lexical and pre-lexical representations are more reliably activated than are conceptual representations (Holcomb et al., 2005; Schnyer et al., 1997). Conceptual information is activated to a much greater extent during conscious word perception and intentional study than during a masked word presentation. Accordingly, study-phase exposure may have resulted primarily in fluency of the conceptual variety, reflected in frontal N400 potentials; masked priming may have primarily enhanced lexical and pre-lexical forms of fluency, reflected in posterior N400 potentials. Consistent with this interpretation are prior findings linking FN400 potentials to conceptual priming (Voss & Paller, 2006; Voss et al., 2010b), along with evidence that posterior N400 potentials are sensitive to factors that facilitate lexical and pre-lexical processing in addition to conceptual processing (for review, see Kutas and Federmeier, 2011).
On the other hand, the relationship between FN400 and N400 ERPs is poorly understood at present, and alternate accounts of this relationship warrant consideration (e.g., Voss & Federmeier, 2011). For example, N400-like effects in lexical decision and sentence verification tasks have been found to be more anterior for concrete words than for abstract words (Kounios & Holcomb, 1994; Holcomb, Kounios, Anderson, & West, 1999; West & Holcomb, 2000). More anterior effects have also been found on these tasks when pictures were used as stimuli (McPherson & Holcomb, 1999). Holcomb et al. (1999) thus suggested that there is an anterior “imagistically sensitive N400” which is activated relatively more by concrete words and pictures, as well as a posterior “linguistically sensitive” N400 that is not affected by imagery. The present findings can be interpreted in light of these ideas if one assumes that the use of mental imagery as an encoding strategy at study led to facilitated imagistic processing at test for K Hits in Experiment 1, whereas no robust imagistic facilitation resulted from masked priming. Interestingly, Lee and Federmeier (2008) observed that these frontal “concreteness effects” on ERPs sometimes extend beyond the latency window typically ascribed to N400 potentials, lasting from ~300 to 800 ms. The frontal difference between K hits and misses in Experiment 1 remained significant through this longer time window, further hinting at a parallel between these frontal ERPs and those related in other contexts to imagery or concreteness. Future research will be necessary to arbitrate on these issues, including direct comparisons of priming manipulations that are lexical versus conceptual in nature, as well as of situations in which recognition memory has relatively more or less potential to benefit from imagistic stimulus processing.
Importantly, although the relationship between FN400 and N400 potentials is an active topic of research, these data suggest that neither ERP is related to familiarity in a generic sense. Rather, both reflect one or more specific precursors. A key implication of these findings is thus that familiarity is multiply determined on a neural level, such that the neural measures that co-vary with conscious familiarity experiences depend upon the source or sources that are operative. Other fluency-driven phenomena, such as priming, can occur at many levels of abstraction and in association with a wide variety of neural signals. It thus seems plausible that the neural basis of familiarity is similarly heterogeneous7. Finding that a particular fluency signal can be dissociated from conscious recognition experiences in one situation does not, thereby, imply that these memory phenomena are inherently or immutably independent. Indeed, N400 potentials have been linked to word repetition without awareness—and were dissociated from other ERPs that correlated with familiarity and recollection—in previous studies of recognition memory (e.g., Rugg, Mark, Walla, Schloerscheidt, Birch, & Allan, 1998; Yu & Rugg, 2010). These prior findings, taken together with the current research, suggest that fluency signals that operate outside of consciousness in one situation can interact with conscious memory expressions in another.
This realization highlights a limitation of the field’s strong focus on the use of neural dissociations to characterize conscious and nonconscious memory phenomena. This focus is apparent not only in the ERP literature reviewed here, but also within the relevant fMRI literature, much of which posits that recollection and familiarity are implemented by computations within the hippocampus and perirhinal cortex, respectively, while posterior neocortical regions compute most forms of fluency. By contrast, other recent accounts propose that patterns of connectivity among these and other brain regions can better describe mnemonic behaviors than can patterns of localized neural activity (e.g., Henson & Gagnepain, 2010; Mayes & Roberts, 2001). Thus, while each of these brain regions may differ in terms of the content or complexity of the information it represents, in most cases behavior will be determined by complex and dynamic patterns of communication among many of these regions. The present findings emphasize that one benefit of a highly interactive model is that it can accommodate flexibility in the relationship of particular memory signals to both conscious states and behavioral outcomes (see also Cowell, Bussey, & Saksida, 2010). Indeed, analyses of the functional connectivity between brain regions have revealed interactions between memory phenomena that are not apparent in local patterns of activity, including novel interactions between priming and recollection (Gagnepain et al. 2011). It is likely that measurements of connectivity can also be harnessed to further enhance understanding of relationships between familiarity and multiple forms of fluency.
This recommended shift in focus to a multiply determined account of familiarity would serve to productively re-direct current controversies regarding putative neural correlates of familiarity. For example, findings that FN400 potentials generally correlate with familiarity for meaningful but not meaningless stimuli have sparked a polarizing debate over whether these ERPs should be assigned to familiarity or to conceptual fluency (c.f. Paller et al. 2007; Rugg & Curran, 2007). The present findings call for a movement of this discussion toward ways in which a multitude of neural signals—including, but not limited to FN400—may relate to both familiarity and certain forms of fluency. Indeed, it would seem illogical to assign the posterior N400 potentials identified in this study exclusively to only one of these memory phenomena. Rather, these potentials likely reflected a situation wherein across-trial variations in fluency induced by the masked primes gave rise to across-trial variations in familiarity. Analogously, it seems plausible that in laboratory studies of recognition memory—which typically employ meaningful stimuli such as words or familiar objects—some stimuli presented during a study phase may receive relatively large amounts of conceptual elaboration at study relative to other stimuli. As a result, when these items are presented for a second time at test, those that received more elaboration would have more conceptual fluency than would those that received less elaboration, and this differential fluency could, in turn, influence participants’ likelihood of experiencing these items as familiar (see Voss & Federmeier, 2011, for a similar argument). In this way, study-phase evoked conceptual fluency could routinely serve as a precursor to familiarity in studies of recognition memory, resulting in a coupling between FN400 potentials and familiarity.
As an important caveat, we do not suggest that fluency and familiarity should be directly equated, even in situations in which the latter is derived from the former. The extent to which a given amount of fluency is attributed to and experienced as familiarity is likely to depend on a variety of factors, most notably whether this fluency exceeds the amount that would be expected for a given stimulus within a given context (Westerman et al., 2002, 2003; Whittlesea, Jacoby, & Girard, 1990) and whether participants are encouraged to attribute fluency to prior exposure as opposed to making a non-mnemonic attribution (e.g., Mayes et al., 1997; Oppenheimer, 2008; Alter & Oppenheimer, 2009). When expectations regarding fluency are high—such as when a word is exceedingly common or is encountered in a congruent context—or when an alternate explanation for fluency such as fame or liking is provided, feelings of familiarity tend not to be produced despite the presence of fluency. Sharp dissociations between priming and recognition memory have been also documented in patients with severe amnesia (e.g., Hamann & Squire, 1997; Levy, Stark, & Squire, 2004; Reber & Squire, 1999; Stark & Squire, 2000; Wagner, Gabrieli, & Verfaellie, 1997), demonstrating the potential for these forms of memory to operate independently.
We also do not mean to imply that the attribution of fluency to prior experience is necessarily the only means by which familiarity experiences can be generated. Rather, there may be circumstances in which familiarity arises from processes that are relatively unrelated to fluency (for example, from the active retrieval of item information from memory; though see Mayes et al., 1997, for a discussion of ways in which active retrieval may work in tandem with fluency-related processes to produce memory experiences). For these reasons, the linear relationship between fluency and familiarity depicted in Fig. 6a belies the complexity that would be demanded of a comprehensive neurocognitive account of familiarity and its relationship to fluency. The factors that govern the translation of fluency into familiarity will be an important topic for future research, as will investigations into when and how familiarity may stem from sources other than fluency.
Finally, it is worth noting that, although the notion that recognition memory can stem from the same fluency sources that underlie implicit memory has received extensive behavioral support (e.g., Jacoby & Dallas, 1981; Jacoby & Whitehouse, 1989; Parkin et al., 2001; Rajaram & Geraci, 2000; Whittlesea et al., 1990), converging neural data have thus far been scarce and mostly indirect (e.g., Voss et al., 2009; Wang et al., 2010). It is perhaps for this reason that many relevant neuroimaging investigations continue to be designed and interpreted under the assumption that neural measures that relate to conscious and nonconscious repetition effects are largely independent (e.g., Rugg & Curran, 2007; Stenberg, Hellman, Johansson, & Rosen, 2009; Woodruff, Hayama, & Rugg, 2006; Yu & Rugg, 2010). Indeed, the presence or absence of covariance of a certain neural measure with the conscious experience of remembering (e.g., as assessed by comparing hits with misses or false alarms with correct rejections) remains a common benchmark for arbitrating between neural measures of implicit and explicit memory phenomena in many studies. The present data strongly caution against this approach by providing some of the clearest and most direct evidence to date that neural measures of implicit fluency can vary in tandem with subjective reports of memory strength. The relationship between the neural basis of recognition memory and implicit memory is thus more complex than can be captured by relying on a simplistic conscious/nonconscious duality, and care should be taken to avoid making assumptions that impede our ability to understand these complexities.
Acknowledgements
We thank Joel Voss for helpful discussions regarding these data. This material is based on work supported by the National Science Foundation under Grant BCS-0818912 and the UK Medical Research Council (MC_A060_5PR10).
Footnotes
Although this procedure has typically been employed with the intention to enhance perceptual fluency (e.g., Huber, Clark, Curran, & Winkielman, 2008; Kurilla & Westerman, 2008; Westerman, 2008; Westerman et al. 2002, 2003; Willems, Germain, Salmon, & Van der Linden, 2009), the extent to which conceptual fluency is enhanced by the matching masked prime words is unclear. With paradigms used to assess performance on lexical decision and other priming tasks following masked priming, effects tended to be more robust and reliable on lexical and pre-lexical levels than on semantic levels (Holcomb, Reder, Misra, & Grainger, 2005; Schnyer, Allen, & Forster, 1997). A likely generalization, then, is that effects of masked repetition priming on recognition memory in large part reflect fluency at pre-conceptual levels.
During post-experiment questioning and debriefing, eight participants indicated that they had some awareness or suspicion that words may have been presented during the “flickers,” and three of these eight participants indicated that they were able to read one or more of the words. However, an increase in “old” responses for MP-same relative to MP-different words is not readily attributable to awareness of masked priming, because awareness of the “true” source of fluency for MP-same words has been found to lead to discounting of this fluency as a cue for recognition, such that participants are less likely to make “old” responses for these trials (Jacoby & Whitehouse, 1989). Awareness of some masked words in some participants is thus unlikely to be responsible for the effects of fluency on recognition decisions reported here.
Concerns have been raised about the use of raw proportions of binary “Remember” and “Know” responses in statistical analyses, given that experiences of recollection and familiarity may not be mutually exclusive. Insofar as recollected items can also be familiar, raw proportions of “Know” responses may underestimate the likelihood that an item evoked familiarity. A common correction is to use the independence remember-know procedure (Yonelinas, 2002), in which familiarity is estimated based on the likelihood of an item receiving a “Know” response given that it did not receive a “Remember” response [Familiarity=P(“Know”)/(l -P(“Remember”))]. Analyses with familiarity computed in this manner produced results that were similar to those obtained using raw proportions. The 2 (study status: studied/unstudied) × 2 (response type: R/K) × 2 (masked priming: MP-same/MP-different) ANOVA yielded a main effect of masked priming [F(1,19) = 4.8, p = .04] with no significant interactions [all p’s > .22]. In addition, an exploratory 2 (study status: studied/unstudied) × 2 (masked priming: MP-same/MP-different) ANOVA on corrected “Know” responses revealed a marginal effect of masked priming [F(1,19) = 3.34, p = .08] and a nonsignificant study status x masked priming interaction [F(1,19) = 1.92, p = .18].
Note that interactions involving confidence should be interpreted with caution given that the two confidence levels were not independent. Because participants could choose only one confidence level per trial, any factor that increases high confidence responding will necessarily decrease low confidence responding, potentially biasing the statistical outcome of interactions involving confidence.
This null interaction may at first seem surprising, given that one might expect the effect of masked priming on false recognition to be selective to items that received a priming-related boost in fluency (i.e., to MP-same items). However, natural across-trial variations in fluency at every level of processing are ubiquitous and occur regardless of whether fluency is experimentally manipulated (for review, see Alter and Oppenheimer, 2009). It is therefore plausible that the heightened salience of certain types of fluency induced by masked priming led some participants to monitor across-trial differences in such fluency for MP-different unstudied items. As such, false recognition for MP-different and MP-same items in this context would be expected to be driven by similar neurocognitive processes.
Because the behavioral effect of masked priming on false alarms was found to be selective to high-confidence responses, we re-ran this key paired comparison after excluding low-confidence false alarms for the 15 participants for whom at least 15 high-confidence MP-same false alarm trials were available. The difference between MP-same high-confidence false alarms and MP-same correct rejections was significant in this participant subgroup [t(14) = 3.42, p = .004].
An allied question is whether the neural mechanisms that support recollection can also support familiarity. Although the present experiments have not tackled this question, it is worth noting that when familiarity has been examined for complex meaningless stimuli—such as squiggles, pseudowords, and unfamiliar faces—qualitatively similar ERPs have been found in conjunction with both familiarity and recollection (Voss & Paller, 2007; Voss et al., 2010a; Yovel & Paller, 2004), and such effects have even been found to vary continuously with familiarity confidence (Voss & Paller, 2009b). Thus, the possibility that experiences of familiarity can be derived from a subset of the same underlying sources as recollection should not be ruled out.
References
- Aggleton JP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends in Cognitive Sciences. 2006;10:455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Alter AL, Oppenheimer DM. Uniting the tribes of fluency to form a metacognitive nation. Personality and Social Psychology Review. 2009;13:219–235. doi: 10.1177/1088868309341564. [DOI] [PubMed] [Google Scholar]
- Brown MEJ, Bodner GE. Re-examining dissociations between remembering and knowing: binaryjudgments vs. independent ratings. Journal of Memory and Language. 2011;65:98–108. [Google Scholar]
- Cleary AM. Orthography, phonology, and meaning: word features that give rise to feelings of familiarity in recognition. Psychonomic Bulletin and Review. 2004;11:446–451. doi: 10.3758/bf03196593. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC psycholinguistic database. The Quarterly Journal of Experimental Psychology. 1981;33:497–505. [Google Scholar]
- Cowell RA, Bussey TJ, Saksida LM. Components of recognition memory: dissociable cognitive processes or just differences in representational complexity? Hippocampus. 2010;20:1245–1262. doi: 10.1002/hipo.20865. [DOI] [PubMed] [Google Scholar]
- Danker JF, Hwang GM, Gauthier L, Geller A, Kahana MJ, Sekuler R. Characterizing the ERP old-new effect in a short-term memory task. Psychophysiology. 2008;45:784–793. doi: 10.1111/j.1469-8986.2008.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnepain P, Henson RNA, Chetelat G, Desgranges B, Lebreton K, Eustache F. Is neocortical-hippocampal connectivity a better predictor of subsequent recollection than local increases in hippocampal activity? New insights on the role of priming. Journal of Cognitive Neuroscience. 2011;23:391–403. doi: 10.1162/jocn.2010.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann SB, Squire LR. Intact perceptual memory in the absence of conscious memory. Behavioral Neuroscience. 1997;111:850–854. doi: 10.1037//0735-7044.111.4.850. [DOI] [PubMed] [Google Scholar]
- Henson RNA. Neuroimaging studies of priming. Progress in Neurobiology. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Gagnepain P. Predictive, interactive multiple memory systems. Hippocampus. 2010;20:1315–1326. doi: 10.1002/hipo.20857. [DOI] [PubMed] [Google Scholar]
- Hintzman DL. Judgments of frequency and recognition memory in a multiple-trace memory model. Psychological Review. 1988;95:528–551. [Google Scholar]
- Holcomb PJ, Grainger J. On the time course of visual word recognition: an event-related potential investigation using masked repetition priming. Journal of Cognitive Neuroscience. 2006;18:1631–1643. doi: 10.1162/jocn.2006.18.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb PJ, Grainger J. Exploring the temporal dynamics of visual word recognition in the masked repetition priming paradigm using event-related potentials. Brain Research. 2007;1180:39–58. doi: 10.1016/j.brainres.2007.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb PJ, Kounios J, Anderson JE, West WC. Dual coding, context-availability, and concreteness effects in sentence comprehension: an electrophysiological investigation. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:721–742. doi: 10.1037//0278-7393.25.3.721. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, Reder L, Misra M, Grainger J. The effects of prime visibility on ERP measures of masked priming. Cognitive Brain Research. 2005;24:155–172. doi: 10.1016/j.cogbrainres.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber DE, Clark TF, Curran T, Winkielman P. Effects of repetition priming on recognition memory: testing a perceptual fluency-disfluency model. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:1305–1324. doi: 10.1037/a0013370. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Whitehouse K. An illusion of memory: false recognition influenced by unconscious perception. Journal of Experimental Psychology: General. 1989;118:126–135. [Google Scholar]
- Jacoby LL, Dallas M. On the relationship between autobiographical memory and perceptual learning. Journal of Experimental Psychology. General. 1981;110:306–340. doi: 10.1037//0096-3445.110.3.306. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Kelley CM, Dywan J. Memory attributions. In: Roediger HL, III FIM, editors. Varieties of memory and consciousness: essays in honour of Endel Tulving. Hillsdale, NJ: Erlbaum; 1989. pp. 391–422. [Google Scholar]
- Kounios J, Holcomb PJ. Concreteness effects in semantic processing: ERP evidence supporting dual-coding theory. Journal of Experimental Psychology: Learning Memory, and Cognition. 1994;20:804–823. doi: 10.1037//0278-7393.20.4.804. [DOI] [PubMed] [Google Scholar]
- Kurilla BP, Westerman DL. Processing fluency affects subjective claims of recollection. Memory and Cognition. 2008;36:82–92. doi: 10.3758/mc.36.1.82. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annual Review of Psychology. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CL, Federmeier KD. To watch, to see, and to differ: an event-related potential study of concreteness effects as a function of word class and lexical ambiguity. Brain and Language. 2008;104:145–158. doi: 10.1016/j.bandl.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DA, Stark CEL, Squire LR. Intact conceptual priming in the absence of declarative memory. Psychological Science. 2004;15:680–686. doi: 10.1111/j.0956-7976.2004.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler G. Recognizing: the judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Mayes AR, Gooding PA, van Eijk R. A new theoretical framework for explicit and implicit memory. Psyche. 1997;3:1–43. [Google Scholar]
- Mayes AR, Roberts N. Theories of episodic amnesia. Philosophical Transactions of the Royal Society, London B. 2001;356:1395–1408. doi: 10.1098/rstb.2001.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: an ambiguity associated with analysis of variance models. Electroencephalography and Clinical Neurophysiology. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- McPherson WB, Holcomb PJ. An electrophysiological investigation of semantic priming with pictures of real objects. Psychophysiology. 1999;36:53–65. doi: 10.1017/s0048577299971196. [DOI] [PubMed] [Google Scholar]
- Meyer P, Mecklinger A, Friederici AD. Bridging the gap between the semantic N400 and the early old/new memory effect. Neuroreport. 2007;18:1009–1013. doi: 10.1097/WNR.0b013e32815277eb. [DOI] [PubMed] [Google Scholar]
- Miller JK, Lloyd ME, Westerman DL. When does modality matter? Perceptual versus conceptual fluency-based illusions in recognition memory. Journal of Memory and Language. 2008;58:1080–1094. [Google Scholar]
- Norman KA. How hippocampus and cortex contribute to recognition memory: revisiting the complementary learning systems model. Hippocampus. 2010;20:1217–1227. doi: 10.1002/hipo.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer DM. The secret life of fluency. Trends in Cognitive Sciences. 2008;12:237–241. doi: 10.1016/j.tics.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Paller KA, Voss JL, Boehm SG. Validating neural correlates of familiarity. Trends in Cognitive Sciences. 2007;11:243–250. doi: 10.1016/j.tics.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Ward J, Squires EJ, Furbear H, Clark A, Townshend J. Data-driven recognition memory: a new technique and some data on age differences. Psychonomic Bulletin and Review. 2001;8:812–819. doi: 10.3758/bf03196222. [DOI] [PubMed] [Google Scholar]
- Rajaram S. Remembering and knowing: two means of access to the personal past. Memory and Cognition. 1993;21:89–102. doi: 10.3758/bf03211168. [DOI] [PubMed] [Google Scholar]
- Rajaram S, Geraci L. Conceptual fluency selectively influences knowing. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:1070–1074. doi: 10.1037//0278-7393.26.4.1070. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Relaxing decision criteria does not improve recognition memory in amnesic patients. Memory and Cognition. 1999;27:501–511. doi: 10.3758/bf03211544. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Sciences. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Mark RE, Walla P, Schloerscheidt AM, Birch CS, Allan K. Dissociation of the neural correlates of implicit and explicit memory. Nature. 1998;392:595–598. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Current Opinion in Neurobiology. 2007;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Allen JJB, Forster KI. Event-related brain potential examination of implicit memory processes: masked and unmasked repetition priming. Neuropsychology. 1997;11:243–260. doi: 10.1037//0894-4105.11.2.243. [DOI] [PubMed] [Google Scholar]
- Shiffrin RM, Steyvers M. A model for recognition memory: REM-retrieving effectively from memory. Psychonomic Bulletin and Review. 1997;4:145–166. doi: 10.3758/BF03209391. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. “Remember” source memory ROCs indicate recollection is a continuous process. Memory. 2010;18:27–39. doi: 10.1080/09658210903390061. [DOI] [PubMed] [Google Scholar]
- Stark CEL, Squire LR. Recognition memory and familiarity judgments in severe amnesia: no evidence for a contribution of repetition priming. Behavioral Neuroscience. 2000;114:459–467. doi: 10.1037//0735-7044.114.3.459. [DOI] [PubMed] [Google Scholar]
- Stenberg G, Hellman J, Johansson M, Rosen I. Familiarity or conceptual priming: event-related potentials in name recognition. Journal of Cognitive Neuroscience. 2009;21:447–460. doi: 10.1162/jocn.2009.21045. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Henson RNA. Could masked conceptual primes increase recollection? The subtleties of measuring recollection and familiarity in recognition memory. doi: 10.1016/j.neuropsychologia.2012.07.029. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunney RJ, Fernie G. Repetition priming affects guessing not familiarity. Behavioral and Brain Functions. 2007;3:40–46. doi: 10.1186/1744-9081-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaellie M, Cermak LS. Perceptual fluency as a cue for recognition judgments in amnesia. Neuropsychology. 1999;13:198–205. doi: 10.1037//0894-4105.13.2.198. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Giovanello KS, Keane MM. Recognition memory in amnesia: effects of relaxing response criteria. Cognitive, Affective, and Behavioral Neuroscience. 2001;1:3–7. doi: 10.3758/cabn.1.1.3. [DOI] [PubMed] [Google Scholar]
- Voss JL, Baym CL, Paller KA. Accurate forced-choice recognition without awareness of memory retrieval. Learning and Memory. 2008;15:454–459. doi: 10.1101/lm.971208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Federmeier KD. FN400 potentials are functionally identical to N400 potentials and reflect semantic processing during recognition testing. Psychophysiology. 2011;48:532–546. doi: 10.1111/j.1469-8986.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Hauner KKY, Paller KA. Establishing a relationship between activity reduction in human perirhinal cortex and priming. Hippocampus. 2009;19:773–778. doi: 10.1002/hipo.20608. [DOI] [PubMed] [Google Scholar]
- Voss JL, Lucas HD, Paller KA. Conceptual priming and familiarity: different expressions of memory during recognition testing with distinct neurophysiological correlates. Journal of Cognitive Neuroscience. 2010a;22:2651–2683. doi: 10.1162/jocn.2009.21341. [DOI] [PubMed] [Google Scholar]
- Voss JL, Lucas HD, Paller KA. More than a feeling: pervasive influences of memory processing without awareness of remembering. Cognitive Neuroscience. 2012;3:193–207. doi: 10.1080/17588928.2012.674935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Fluent conceptual processing and explicit memory for faces are electrophysiologically distinct. The Journal of Neuroscience. 2006;26:926–933. doi: 10.1523/JNEUROSCI.3931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Neural correlates of conceptual implicit memory and their contamination of putative neural correlates of explicit memory. Learning and Memory. 2007;14:259–267. doi: 10.1101/lm.529807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. An electrophysiological signature of unconscious recognition memory. Nature Neuroscience. 2009a;12:349–355. doi: 10.1038/nn.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Remembering and knowing: electrophysiological distinctions at encoding but not retrieval. Neurolmage. 2009b;46:280–289. doi: 10.1016/j.neuroimage.2009.01.048. [DOI] [PubMed] [Google Scholar]
- Voss JL, Paller KA. What makes recognition without awareness appear to be elusive? Strategic factors that influence the accuracy of guesses. Learning and Memory. 2010;17:460–468. doi: 10.1101/lm.1896010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Schendan HE, Paller KA. Finding meaning in novel geometric shapes influences electrophysiological correlates of repetition and dissociates perceptual and conceptual priming. Neurolmage. 2010b;49:2879–2889. doi: 10.1016/j.neuroimage.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Gabrieli JDE, Verfaellie M. Dissociations between familiarity processes in explicit recognition and implicit perceptual memory. Journal of Experimental Psychology: Learning Memory, and Cognition. 1997;23:305–323. doi: 10.1037//0278-7393.23.2.305. [DOI] [PubMed] [Google Scholar]
- Wang W, Lazzara MM, Ranganath C, Knight RT, Yonelinas AP. The medial temporal lobe supports conceptual implicit memory. Neuron. 2010;68:835–842. doi: 10.1016/j.neuron.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West WC, Holcomb PJ. Imaginal, semantic, and surface-level processing of concrete and abstract words: an electrophysiological investigation. Journal of Cognitive Neuroscience. 2000;12:1024–1037. doi: 10.1162/08989290051137558. [DOI] [PubMed] [Google Scholar]
- Westerman DL. Relative fluency and illusions of recognition memory. Psychonomic Bulletin and Review. 2008;15:1196–1200. doi: 10.3758/PBR.15.6.1196. [DOI] [PubMed] [Google Scholar]
- Westerman DL, Lloyd ME, Miller JK. The attribution of perceptual fluency in recognition memory: the role of expectation. Journal of Memory and Language. 2002;47:607–617. [Google Scholar]
- Westerman DL, Miller JK, Lloyd ME. Change in perceptual form attenuates the use of the fluency heuristic in recognition. Memory and Cognition. 2003;31:619–629. doi: 10.3758/bf03196102. [DOI] [PubMed] [Google Scholar]
- Whittlesea BWA, Jacoby LL, Girard K. Illusions of immediate memory: evidence of an attributional basis for feelings of familiarity and perceptual quality. Journal of Memory and Language. 1990;29:716–732. [Google Scholar]
- Willems S, Germain S, Salmon E, Van der Linden M. Patients with Alzheimer’s disease use metamemory to attenuate the Jacoby-Whitehouse illusion. Neuropsychologia. 2009;47:2672–2676. doi: 10.1016/j.neuropsychologia.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychological Review. 2007;114:152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Mickes L, Squire LR. Measuring recollection and familiarity in the medial temporal lobe. Hippocampus. 2010;20:1195–1205. doi: 10.1002/hipo.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff CC, Hayama HR, Rugg MD. Electrophysiological dissociation of the neural correlates of recollection and familiarity. Brain Research. 2006;1100:125–135. doi: 10.1016/j.brainres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Woollams AM, Taylor JR, Karayanidis F, Henson RNA. Event-related potentials associated with masked priming of test cues reveal multiple potential contributions to recognition memory. Journal of Cognitive Neuroscience. 2008;20:1114–1129. doi: 10.1162/jocn.2008.20076. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Parks CM. Receiver operating characteristics (ROCs) in recognition memory: a review. Psychological Bulletin. 2007;133:800–832. doi: 10.1037/0033-2909.133.5.800. [DOI] [PubMed] [Google Scholar]
- Yovel G, Paller KA. The neural basis of the butcher-on-the-bus phenomenon: when a face seems familiar but is not remembered. Neurolmage. 2004;21:789–800. doi: 10.1016/j.neuroimage.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Yu SS, Rugg MD. Dissociation of the electrophysiological correlates of familiarity strength and item repetition. Brain Research. 2010;1320:74–84. doi: 10.1016/j.brainres.2009.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]