Figure 5.
Membrane Curvature Induction by Nup1 and Nup60 in a Reconstituted System
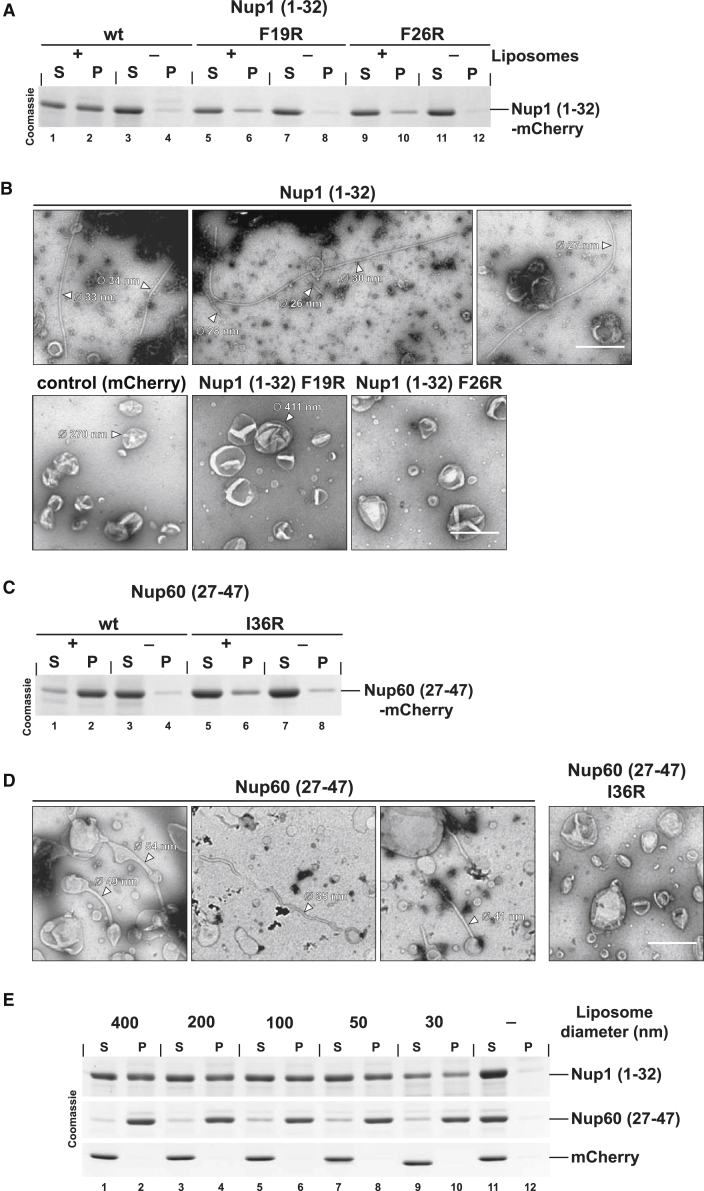
(A) Coomassie-stained gel of a liposome co-sedimentation assay using Folch liposomes (0.5 mg/ml, size-filtered to 400 nm) and Strep-tagged recombinant Nup1 proteins. Liposome bound protein is pelleted upon centrifugation. S, supernatant; P, pellet.
(B) In vitro liposome deformation assay showing a gallery of representative tubule shapes. Folch liposomes were mixed with purified wild-type or mutant Strep-Nup1 (1-32)-mCherry or Strep-mCherry (control) (final protein: 2.5 μM) and incubated at 22°C for 30 min prior to negative staining EM. For a protein control without liposomes, see Figure S6A. Outer diameters of liposomes and tubules are indicated. Scale bar represents 500 nm.
(C) Liposome co-sedimentation assay as in (A). Recombinant wild-type and mutant 6His-Nup60 (27-47)-mCherry protein was used.
(D) In vitro liposome deformation assay with 6His-Nup60 (27-47)-mCherry (final protein: 2.5 μM, 30 min incubation at 22°C) showing different types of tubules that are commonly observed by EM. For a Nup60 protein control without liposomes and further examples of tubule morphologies, see Figure S6C. Tubule diameters are indicated. Scale bar represents 500 nm.
(E) Liposome co-sedimentation assay with recombinant Nup1, Nup60, and mCherry (control) proteins. Liposomes of varying sizes (0.5 mg/ml) were made by extrusion through membranes of different pore diameters (as indicated). The actual liposome diameters were determined by dynamic light scattering (Figure S6D). Pellet and supernatant fractions were analyzed by SDS-PAGE and Coomassie staining.