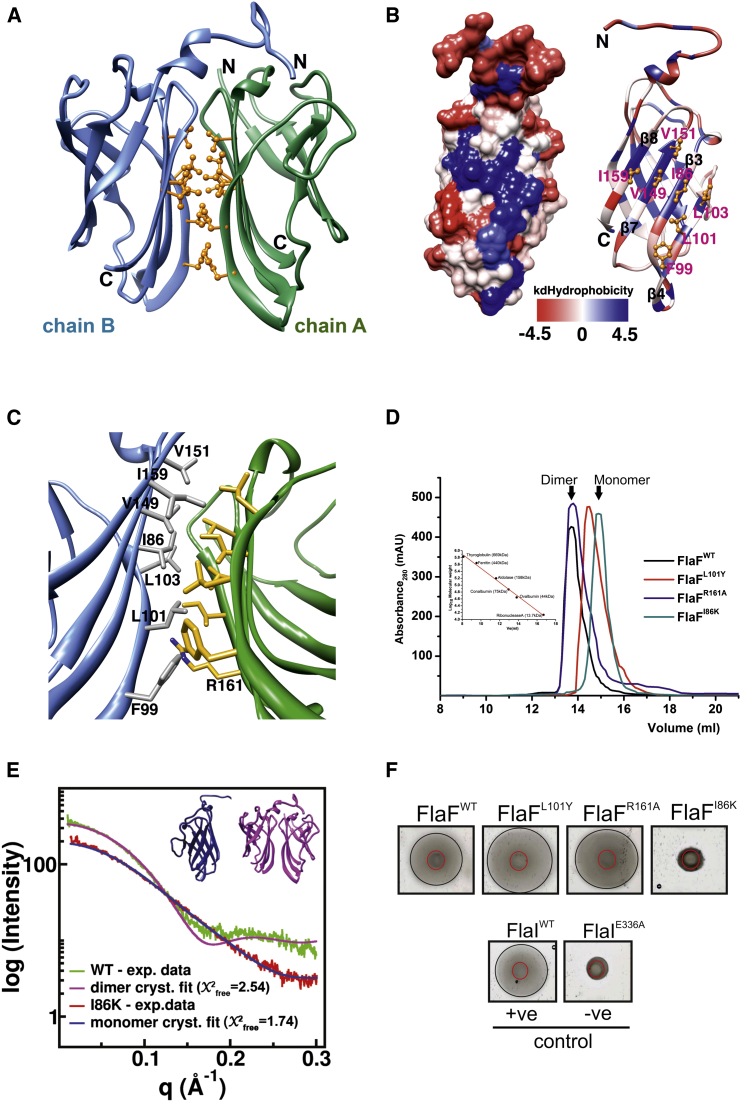
Figure 3.
sFlaF Dimer Formation through Hydrophobic Interactions
(A) The dimeric form of the sFlaF crystal structure. The hydrophobic residues are shown (gold, ball and stick). The dimer interface was between two β-sheets (β3, β4, β7, and β8) from each sFlaF subunit (chain A and B). The two subunits are in a two-fold symmetry.
(B) Left: the hydrophobicity surface presentation was prepared by Chimera. The color gradient ranges from blue (most hydrophobic), through white, to red (most hydrophilic) using the Kyte-DooLittle hydrophobicity scale (Kyte and Doolittle, 1982); right: the ribbon presentation with the corresponding color gradient. The hydrophobic residues (gold, ball and stick) that form a hydrophobic patch (the bluest region) on strand β3, β4, β7, and β8 are labeled in pink.
(C) The close-up view of the sFlaF dimer interface. The interface residues shown in stick are colored in dark gray and gold, respectively. Residue R161 has H-bonding with the main chain carbonyl oxygen of residue L101 (not shown).
(D) Analytical size-exclusion chromatogram of sFlaF dimer interface mutants. sFlaFWT and sFlaF-R161A elute as a homogeneous dimeric population, whereas sFlaF-L101Y contains a mixture of dimeric and monomeric sFlaF in solution. Interestingly, sFlaF-I86K elutes solely as a monomeric population. The size standard is shown in the inset.
(E) The SAXS results show that wild-type (WT)- and I86K-sFlaF exist in solution as dimer and monomer, respectively. The calculated scattering curves of the WT-sFlaF dimer (pink) and monomer (chain B, blue) were fit to the SAXS data of WT-sFlaF (green) and I86K-sFlaF (red) with X2free fit of 2.54 and 1.74, respectively.
(F) Motility assay of in-trans complemented ΔaapFΔFlaF strain using FlaF dimer interface mutants. The results clearly show that monomeric FlaF is not able to complement the deletion defect. The positive (+ve) and negative (−ve) controls used in this study were FlaIWT and FlaI-E336A complemented ΔaapFΔFlaI strains, respectively.