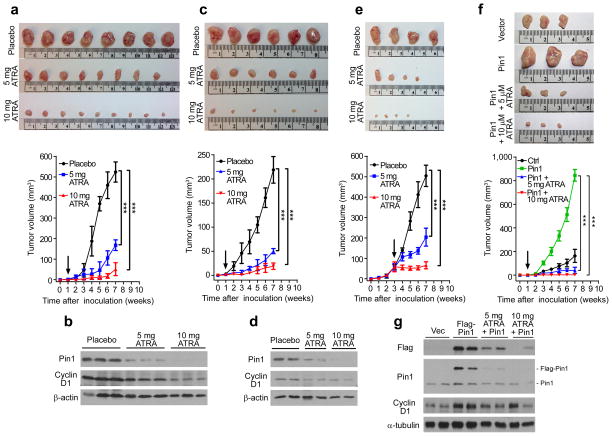
Figure 6. ATRA exerts potent anticancer activity against TNBC in vivo by ablating Pin1 and thereby blocking multiple cancer pathways simultaneously.
(a–d) Nude mice were flank-inoculated with 2 × 106 MDA-MB-231 cells (a and b) or MDA-MB-468 cells (c and d), 1 week later, implanted with 5 or 10 mg 21 day ATRA-releasing or placebo pellets. Tumor sizes were weekly measured and mice were sacrificed after 7 weeks to collect tumor tissues (a, c, upper). Curves of tumor volume were plotted over time (a, c, lower) (mean ± s.d. of eight mice for a, six mice for c). Pin1 and cyclin D1 in xenograft tumors were assayed by IB (b and d).
(e) Nude mice were flank-inoculated with 2 × 106 MDA-MB-231 cells and, 3 week later (arrow), implanted with 5 or 10 mg 21 day ATRA-releasing or placebo pellets. Tumor sizes were weekly measured and mice were sacrificed after 4 weeks to collect tumor tissues (upper). Curves of tumor volume were plotted over time (lower) (mean ± s.d. of four mice).
(f and g) MDA-MB-231 cells stably expressing Flag-Pin1 or control vector were inoculated into nude mice, and 1 week later treated with ATRA implants for 7 weeks before collecting tumors (f, upper) Quantitative curves of tumor volume were plotted (f, lower) (mean ± s.d. of three mice). Exogenous and endogenous Pin1 along cyclin D1 in xenograft tumors were assayed by IB (g).
*P < 0.05, **P < 0.01, ***P < 0.001, as determined by Student’s t-test.