Abstract
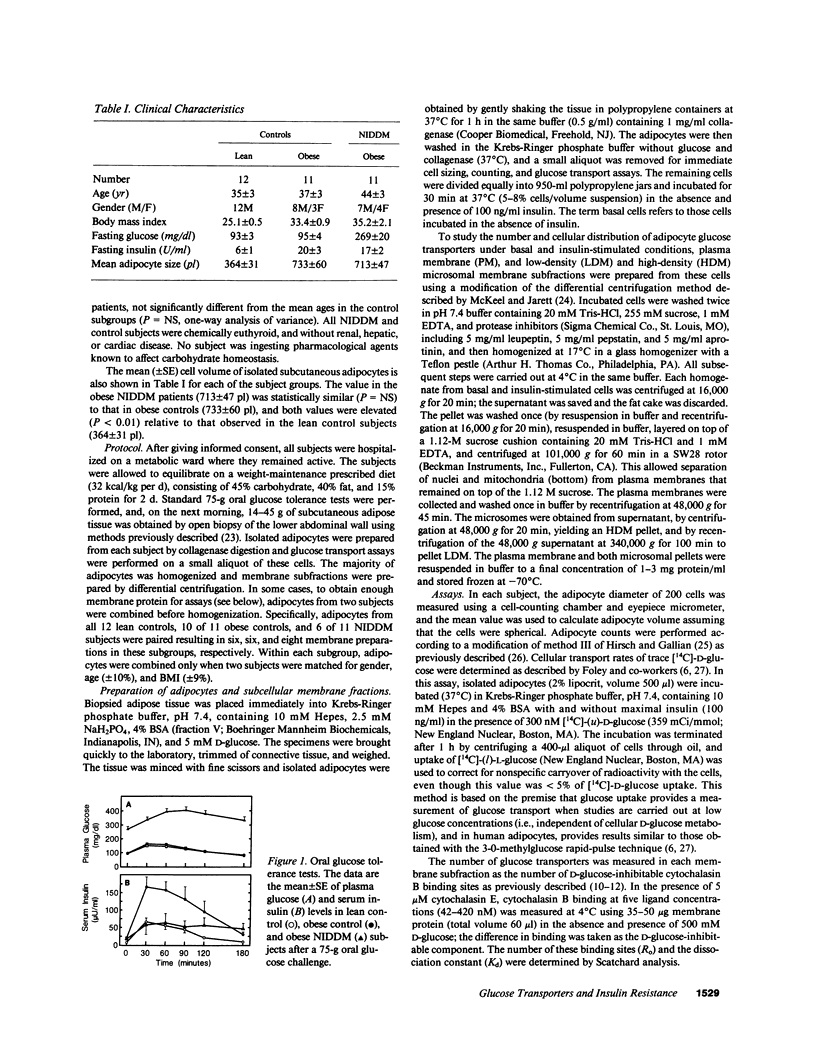
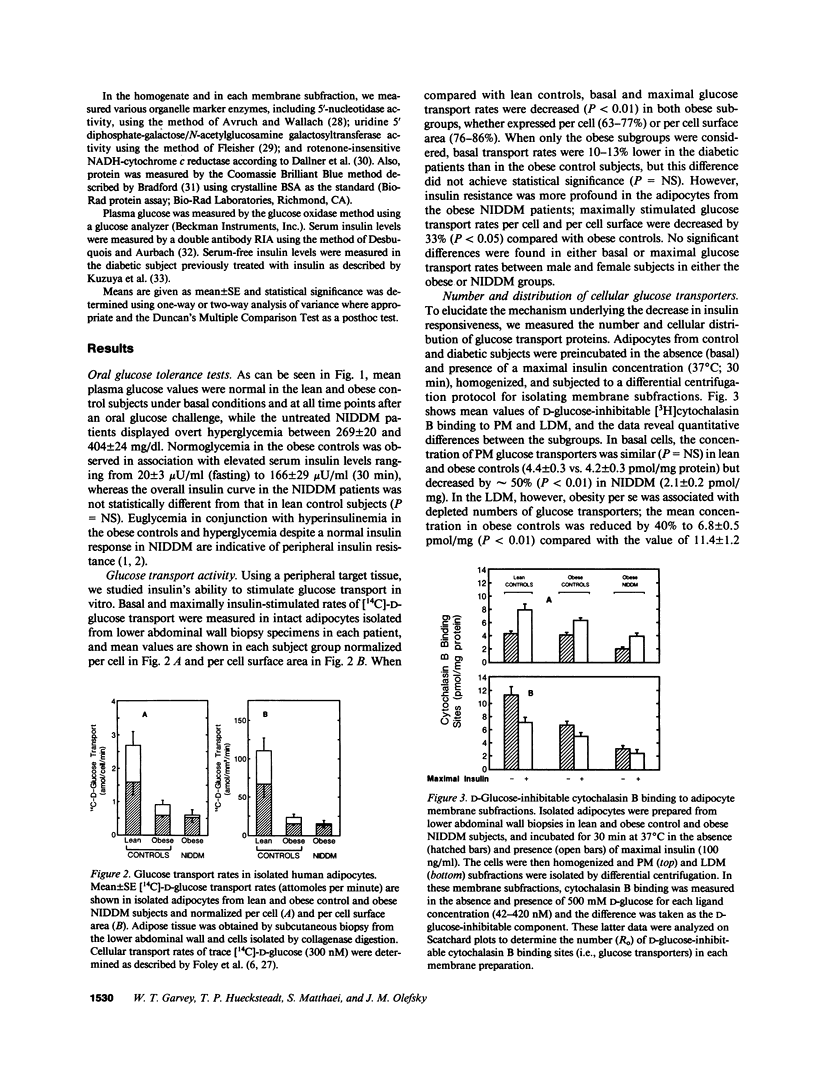
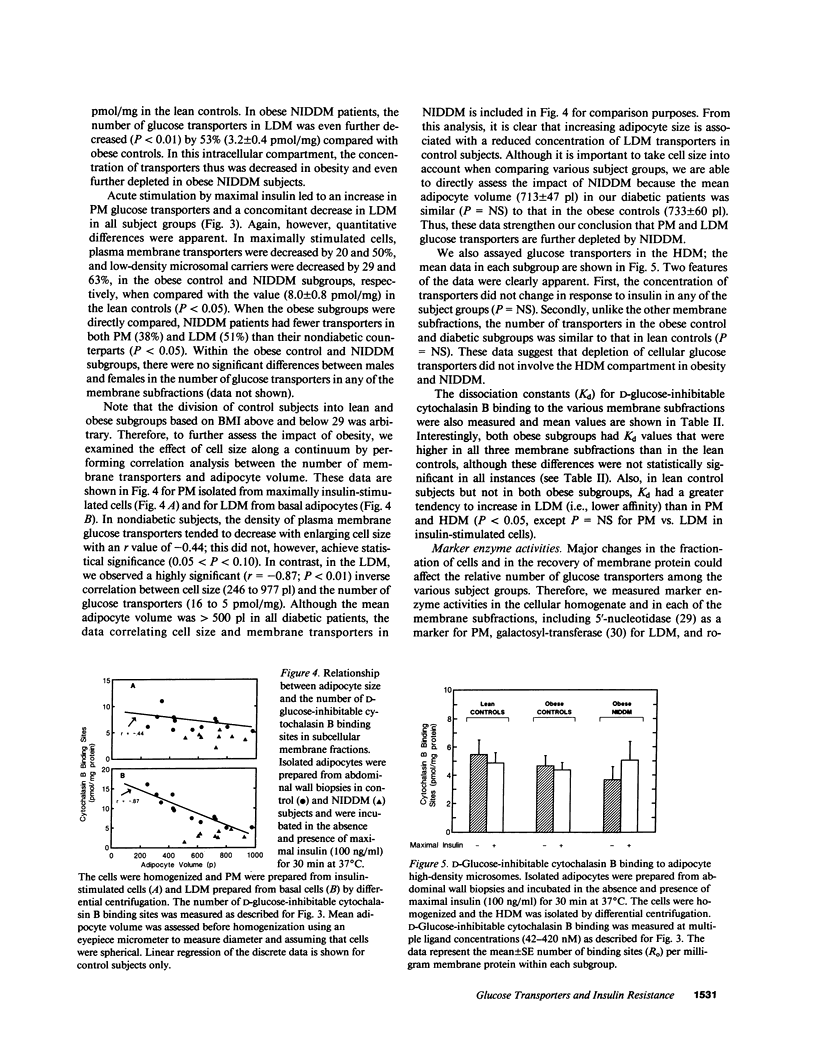
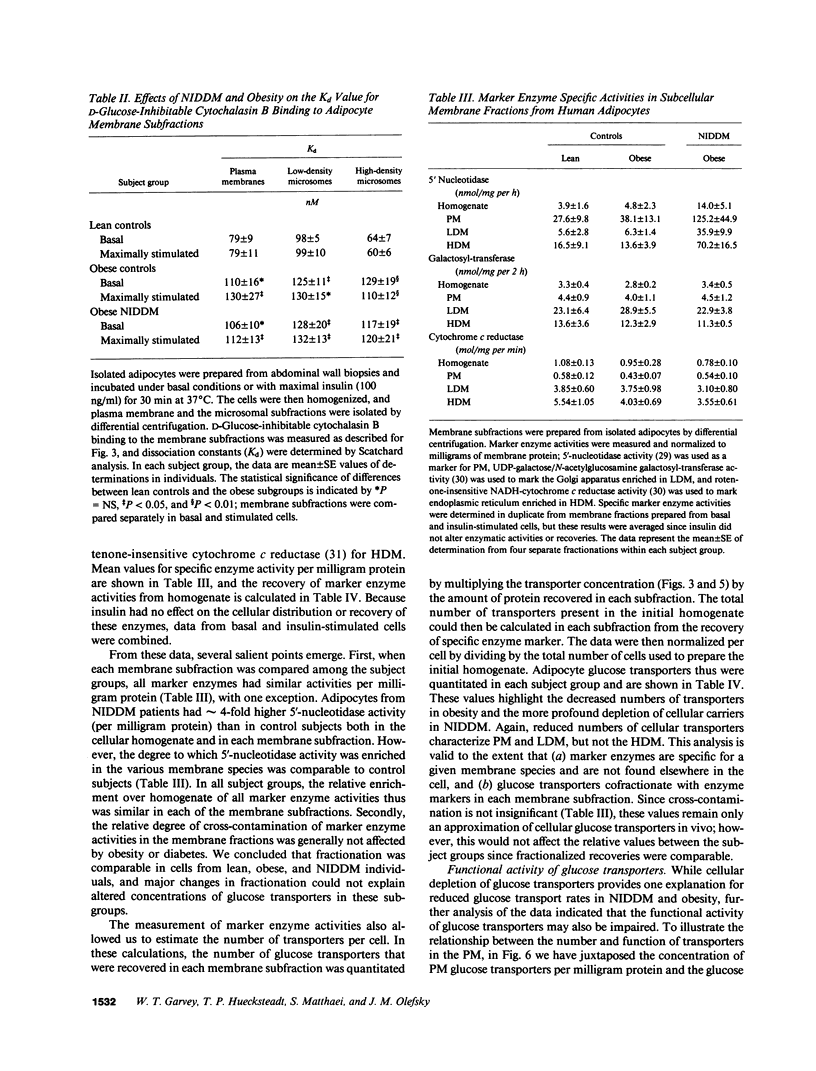
To examine the role of glucose transport proteins in cellular insulin resistance, we studied subcutaneous adipocytes isolated from lean control, obese control (body mass index [BMI] 33.4 +/- 0.9), and untreated obese non-insulin-dependent diabetes mellitus (NIDDM) patients (BMI 35.2 +/- 2.1; fasting glucose 269 +/- 20 mg/dl). Glucose transporters were measured in plasma membrane (PM), low-density (LDM), and high-density (HDM) microsomal subfractions from basal and maximally insulin-stimulated cells using the cytochalasin B binding assay, and normalized per milligram of membrane protein. In all subgroups, insulin led to an increase in PM glucose transporters and a corresponding depletion of transporters in the LDM. Insulin recruited 20% fewer transporters to the PM in the obese subgroup when compared with lean controls, and this was associated with a decline in LDM transporters with enlarging cell size in the control subjects. In NIDDM, PM, and LDM, transporters were decreased 50% in both basal and stimulated cells when compared with obese controls having similar mean adipocyte size. Cellular depletion of glucose transporters was not the only cause of insulin resistance, because the decrease in rates of [14C]-D-glucose transport (basal and insulin-stimulated) was greater than could be explained by reduced numbers of PM transporters in both NIDDM and obesity. In HDM, the number of transporters was not influenced by insulin and was similar in all subgroups. We conclude that (a) in NIDDM and obesity, both reduced numbers and impaired activity of glucose transporters contribute to cellular insulin resistance, and (b) in NIDDM, more profound cellular insulin resistance is associated primarily with a further depletion of cellular transporters.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W. J., Vasquez B., Nagulesparan M., Klimes I., Foley J., Unger R., Reaven G. M. Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984 Jul;33(7):634–642. doi: 10.2337/diab.33.7.634. [DOI] [PubMed] [Google Scholar]
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Bolinder J., Kager L., Ostman J., Arner P. Differences at the receptor and postreceptor levels between human omental and subcutaneous adipose tissue in the action of insulin on lipolysis. Diabetes. 1983 Feb;32(2):117–123. doi: 10.2337/diab.32.2.117. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carter-Su C., Okamoto K. Effect of glucocorticoids on hexose transport in rat adipocytes. Evidence for decreased transporters in the plasma membrane. J Biol Chem. 1985 Sep 15;260(20):11091–11098. [PubMed] [Google Scholar]
- Ciaraldi T. P., Horuk R., Matthaei S. Biochemical and functional characterization of the rat liver glucose-transport system. Comparisons with the adipocyte glucose-transport system. Biochem J. 1986 Nov 15;240(1):115–123. doi: 10.1042/bj2400115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaraldi T. P., Kolterman O. G., Olefsky J. M. Mechanism of the postreceptor defect in insulin action in human obesity. Decrease in glucose transport system activity. J Clin Invest. 1981 Oct;68(4):875–880. doi: 10.1172/JCI110342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaraldi T. P., Kolterman O. G., Scarlett J. A., Kao M., Olefsky J. M. Role of glucose transport in the postreceptor defect of non-insulin-dependent diabetes mellitus. Diabetes. 1982 Nov;31(11):1016–1022. doi: 10.2337/diacare.31.11.1016. [DOI] [PubMed] [Google Scholar]
- Ciaraldi T. P., Kolterman O. G., Siegel J. A., Olefsky J. M. Insulin-stimulated glucose transport in human adipocytes. Am J Physiol. 1979 Jun;236(6):E621–E625. doi: 10.1152/ajpendo.1979.236.6.E621. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. II. Synthesis of constitutive microsomal enzymes in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):97–117. doi: 10.1083/jcb.30.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Fleischer B. Isolation and characterization of Golgi apparatus and membranes from rat liver. Methods Enzymol. 1974;31:180–191. doi: 10.1016/0076-6879(74)31020-8. [DOI] [PubMed] [Google Scholar]
- Foley J. E., Kashiwagi A., Verso M. A., Reaven G., Andrews J. Improvement in in vitro insulin action after one month of insulin therapy in obese noninsulin-dependent diabetics. Measurements of glucose transport and metabolism, insulin binding, and lipolysis in isolated adipocytes. J Clin Invest. 1983 Dec;72(6):1901–1909. doi: 10.1172/JCI111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Griffin J., Hamman R. F., Kolterman O. G. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985 Mar;34(3):222–234. doi: 10.2337/diab.34.3.222. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Matthaei S., Marshall S. Glucose and insulin co-regulate the glucose transport system in primary cultured adipocytes. A new mechanism of insulin resistance. J Biol Chem. 1987 Jan 5;262(1):189–197. [PubMed] [Google Scholar]
- Haspel H. C., Wilk E. W., Birnbaum M. J., Cushman S. W., Rosen O. M. Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem. 1986 May 25;261(15):6778–6789. [PubMed] [Google Scholar]
- Hebert D. N., Carruthers A. Direct evidence for ATP modulation of sugar transport in human erythrocyte ghosts. J Biol Chem. 1986 Aug 5;261(22):10093–10099. [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- Joost H. G., Weber T. M., Cushman S. W., Simpson I. A. Insulin-stimulated glucose transport in rat adipose cells. Modulation of transporter intrinsic activity by isoproterenol and adenosine. J Biol Chem. 1986 Aug 5;261(22):10033–10036. [PubMed] [Google Scholar]
- Karnieli E., Barzilai A., Rafaeloff R., Armoni M. Distribution of glucose transporters in membrane fractions isolated from human adipose cells. Relation to cell size. J Clin Invest. 1986 Oct;78(4):1051–1055. doi: 10.1172/JCI112660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnieli E., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. A possible mechanism of insulin resistance in the rat adipose cell in streptozotocin-induced diabetes mellitus. Depletion of intracellular glucose transport systems. J Clin Invest. 1981 Sep;68(3):811–814. doi: 10.1172/JCI110318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnieli E., Zarnowski M. J., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981 May 25;256(10):4772–4777. [PubMed] [Google Scholar]
- Kashiwagi A., Huecksteadt T. P., Foley J. E. The regulation of glucose transport by cAMP stimulators via three different mechanisms in rat and human adipocytes. J Biol Chem. 1983 Nov 25;258(22):13685–13692. [PubMed] [Google Scholar]
- Kashiwagi A., Verso M. A., Andrews J., Vasquez B., Reaven G., Foley J. E. In vitro insulin resistance of human adipocytes isolated from subjects with noninsulin-dependent diabetes mellitus. J Clin Invest. 1983 Oct;72(4):1246–1254. doi: 10.1172/JCI111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T., Robinson F. W., Blevins T. L., Ezaki O. Evidence that translocation of the glucose transport activity is the major mechanism of insulin action on glucose transport in fat cells. J Biol Chem. 1982 Sep 25;257(18):10942–10947. [PubMed] [Google Scholar]
- Kuroda M., Honnor R. C., Cushman S. W., Londos C., Simpson I. A. Regulation of insulin-stimulated glucose transport in the isolated rat adipocyte. cAMP-independent effects of lipolytic and antilipolytic agents. J Biol Chem. 1987 Jan 5;262(1):245–253. [PubMed] [Google Scholar]
- Kuzuya H., Blix P. M., Horwitz D. L., Steiner D. F., Rubenstein A. H. Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes. 1977 Jan;26(1):22–29. doi: 10.2337/diab.26.1.22. [DOI] [PubMed] [Google Scholar]
- Marshall S., Garvey W. T., Geller M. Primary culture of isolated adipocytes. A new model to study insulin receptor regulation and insulin action. J Biol Chem. 1984 May 25;259(10):6376–6384. [PubMed] [Google Scholar]
- Matthaei S., Garvey W. T., Horuk R., Hueckstaedt T. P., Olefsky J. M. Human adipocyte glucose transport system. Biochemical and functional heterogeneity of hexose carriers. J Clin Invest. 1987 Mar;79(3):703–709. doi: 10.1172/JCI112874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeel D. W., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. J Cell Biol. 1970 Feb;44(2):417–432. doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven G. M., Bernstein R., Davis B., Olefsky J. M. Nonketotic diabetes mellitus: insulin deficiency or insulin resistance? Am J Med. 1976 Jan;60(1):80–88. doi: 10.1016/0002-9343(76)90536-2. [DOI] [PubMed] [Google Scholar]
- Scarlett J. A., Gray R. S., Griffin J., Olefsky J. M., Kolterman O. G. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982 Jul-Aug;5(4):353–363. doi: 10.2337/diacare.5.4.353. [DOI] [PubMed] [Google Scholar]
- Scarlett J. A., Kolterman O. G., Ciaraldi T. P., Kao M., Olefsky J. M. Insulin treatment reverses the postreceptor defect in adipocyte 3-O-methylglucose transport in type II diabetes mellitus. J Clin Endocrinol Metab. 1983 Jun;56(6):1195–1201. doi: 10.1210/jcem-56-6-1195. [DOI] [PubMed] [Google Scholar]
- Smith U., Kuroda M., Simpson I. A. Counter-regulation of insulin-stimulated glucose transport by catecholamines in the isolated rat adipose cell. J Biol Chem. 1984 Jul 25;259(14):8758–8763. [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardzala L. J., Cushman S. W., Salans L. B. Mechanism of insulin action on glucose transport in the isolated rat adipose cell. Enhancement of the number of functional transport systems. J Biol Chem. 1978 Nov 25;253(22):8002–8005. [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Potential mechanism of insulin action on glucose transport in the isolated rat diaphragm. Apparent translocation of intracellular transport units to the plasma membrane. J Biol Chem. 1981 Jul 25;256(14):7090–7093. [PubMed] [Google Scholar]
- Watanabe T., Smith M. M., Robinson F. W., Kono T. Insulin action on glucose transport in cardiac muscle. J Biol Chem. 1984 Nov 10;259(21):13117–13122. [PubMed] [Google Scholar]


