Figure 1.
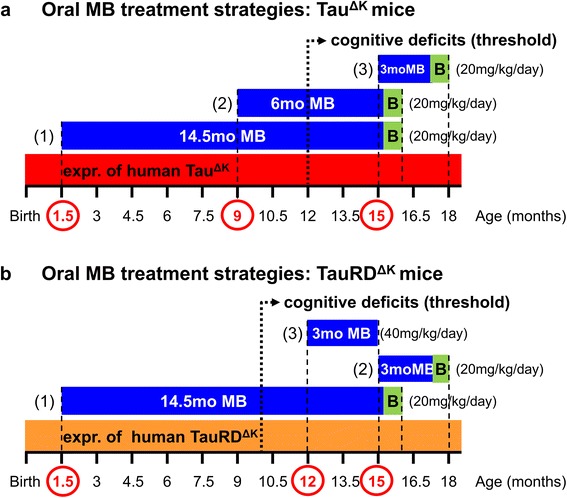
Oral MB treatment strategies of mice. (a) Full-lengthTauΔK mice received MB for 14.5mo starting at 1.5mo of age, before onset of Tau pathological changes and cognitive decline (protocol 1, 1.5 mo Tau pathology, 14.5 mo preventive MB, 20 mg/kg), for 6mo starting at 9mo of age, shortly before the onset of cognitive impairments (protocol 2, 9 mo Tau pathology, 6 mo preventive MB, 20 mg/kg) and for 3mo starting at 15mo of age, at a time point, when learning and memory deficits are already present (protocol 3, 15 mo Tau pathology, 3 mo therapeutic MB, 20 mg/kg). The constant expression of TauΔK throughout the entire life-span in the absence of doxycycline is depicted as red bar. The onset of progressive cognitive failure starting ~12 months of age is represented by the dotted arrow. (b) TauRDΔK received MB for 14.5mo starting at the age of 1.5mo, before onset of Tau pathology and cognitive impairment (protocol 1, 1.5 mo Tau pathology, 14.5 mo preventive MB, 20 mg/kg) and for 3mo starting at 15mo of age, after the onset of cognitive decline (protocol 2, 15mo Tau pathology, 3mo therapeutic MB, 20 mg/kg). An excessive MB dose of 40 mg/kg was applied for 3mo at the age of 12-15mo (protocol 3, excessive MB, 40 mg/kg). The constant expression of TauRDΔK throughout life-span in the absence of doxycycline is depicted as orange bar. The onset of cognitive deficits starting ~10mo of age is represented by the dotted arrow. Periods and initiation of MB treatment are indicated by blue bars and red circles on the time axis, including period of behavioral testing during the final month (green box). The daily MB dose (20 or 40 mg/kg) for each treatment protocol is given in brackets. MB was administered via the drinking water. MB: methylene blue; mo: months; B: behavior tests; expr.: expression.
