Abstract
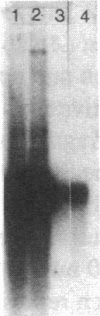
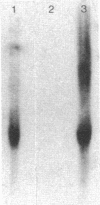
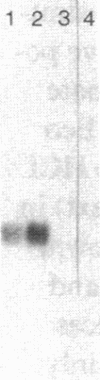
Transfection of an activated rat oncogene into NIH3T3 fibroblasts leads to transformation and induction of a metastatic phenotype. To identify genes whose activation might mediate these processes, we used a differential screening strategy. A 1.5-kb transcript is induced fiftyfold, constitutes 1% of ras transformed cell messenger RNA (mRNA) and is the most abundantly induced message in these cells. Our sequence data shows that it encodes murine cathepsin L, a potent collagenolytic and elastinolytic lysosomal enzyme. The murine clone was used to isolate human cathepsin L complementary DNA (cDNA) clones. The complete nucleotide and deduced amino acid sequences of human and murine preprocathepsin L are presented and compared to other papain family cysteine proteinases. Northern analysis shows that both human and murine cathepsin L probes hybridize to a 1.5-kb transcript in several tissues, but also to a 4-kb transcript in human kidney. These clones will facilitate studies of the structure, expression, and function of cathepsin L, including its unexpected upregulation in transformation.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Graf J., Kitten G. T., Kleinman H. K., Martin G. R., Veillette A., Lippman M. E. 17 beta-estradiol regulates and v-Ha-ras transfection constitutively enhances MCF7 breast cancer cell interactions with basement membrane. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8182–8186. doi: 10.1073/pnas.83.21.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeff M., Slater D. E., Bressler J., Furth M. E. Cellular ras oncogene expression and cell cycle measured by flow cytometry in hematopoietic cell lines. Blood. 1986 Mar;67(3):676–681. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Baumbach G. A., Saunders P. T., Bazer F. W., Roberts R. M. Uteroferrin has N-asparagine-linked high-mannose-type oligosaccharides that contain mannose 6-phosphate. Proc Natl Acad Sci U S A. 1984 May;81(10):2985–2989. doi: 10.1073/pnas.81.10.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. O., Kraynak A. R., Storer R. D., Gibbs J. B. Experimental metastasis in nude mice of NIH 3T3 cells containing various ras genes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5277–5281. doi: 10.1073/pnas.83.14.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickell P. M., Latchman D. S., Murphy D., Willison K., Rigby P. W. Activation of a Qa/Tla class I major histocompatibility antigen gene is a general feature of oncogenesis in the mouse. Nature. 1983 Dec 22;306(5945):756–760. doi: 10.1038/306756a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. G proteins: control of exocytosis. Nature. 1987 Jul 9;328(6126):112–113. doi: 10.1038/328112a0. [DOI] [PubMed] [Google Scholar]
- Capony F., Morisset M., Barrett A. J., Capony J. P., Broquet P., Vignon F., Chambon M., Louisot P., Rochefort H. Phosphorylation, glycosylation, and proteolytic activity of the 52-kD estrogen-induced protein secreted by MCF7 cells. J Cell Biol. 1987 Feb;104(2):253–262. doi: 10.1083/jcb.104.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. J., San Segundo B., McCormick M. B., Steiner D. F. Nucleotide and predicted amino acid sequences of cloned human and mouse preprocathepsin B cDNAs. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7721–7725. doi: 10.1073/pnas.83.20.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Hamilton R. T., Parfett C. L., Edwards D. R., St Pierre R., Waterhouse P., Nilsen-Hamilton M. Close relationship of the major excreted protein of transformed murine fibroblasts to thiol-dependent cathepsins. Cancer Res. 1986 Sep;46(9):4590–4593. [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Kasid A., Huff K. K., Bates S. E., Knabbe C., Bronzert D., Gelmann E. P., Lippman M. E. Activation of growth factor secretion in tumorigenic states of breast cancer induced by 17 beta-estradiol or v-Ha-ras oncogene. Proc Natl Acad Sci U S A. 1987 Feb;84(3):837–841. doi: 10.1073/pnas.84.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan S. E., McClarty G. A., Jarolim L., Wright J. A., Spiro I., Hager G., Greenberg A. H. Expression of H-ras correlates with metastatic potential: evidence for direct regulation of the metastatic phenotype in 10T1/2 and NIH 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):830–837. doi: 10.1128/mcb.7.2.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frick K. K., Doherty P. J., Gottesman M. M., Scher C. D. Regulation of the transcript for a lysosomal protein: evidence for a gene program modified by platelet-derived growth factor. Mol Cell Biol. 1985 Oct;5(10):2582–2589. doi: 10.1128/mcb.5.10.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel C. A., Goldberg D. E., Kornfeld S. Identification and characterization of cells deficient in the mannose 6-phosphate receptor: evidence for an alternate pathway for lysosomal enzyme targeting. Proc Natl Acad Sci U S A. 1983 Feb;80(3):775–779. doi: 10.1073/pnas.80.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal S., Gottesman M. M. The major excreted protein of transformed fibroblasts is an activable acid-protease. J Biol Chem. 1986 Feb 5;261(4):1760–1765. [PubMed] [Google Scholar]
- Gal S., Willingham M. C., Gottesman M. M. Processing and lysosomal localization of a glycoprotein whose secretion is transformation stimulated. J Cell Biol. 1985 Feb;100(2):535–544. doi: 10.1083/jcb.100.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser A. G., Der C. J., Marshall C. J., Stanbridge E. J. Suppression of tumorigenicity with continued expression of the c-Ha-ras oncogene in EJ bladder carcinoma-human fibroblast hybrid cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5209–5213. doi: 10.1073/pnas.83.14.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck S., Cannon C., Al-Awqati Q. Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4327–4331. doi: 10.1073/pnas.79.14.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Sobel M. E. Tumor promoters and Kirsten sarcoma virus increase synthesis of a secreted glycoprotein by regulating levels of translatable mRNA. Cell. 1980 Feb;19(2):449–455. doi: 10.1016/0092-8674(80)90519-x. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M. Transformation-dependent secretion of a low molecular weight protein by murine fibroblasts. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2767–2771. doi: 10.1073/pnas.75.6.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig R. G., Koestler T. P., Trainer D. L., Corwin S. P., Miles L., Kline T., Sweet R., Yokoyama S., Poste G. Tumorigenic and metastatic properties of "normal" and ras-transfected NIH/3T3 cells. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3698–3701. doi: 10.1073/pnas.82.11.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand P. H., Thor A., Wunderlich D., Muraro R., Caruso A., Schlom J. Monoclonal antibodies of predefined specificity detect activated ras gene expression in human mammary and colon carcinomas. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5227–5231. doi: 10.1073/pnas.81.16.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. A., Barrett A. J., Mason R. W. Cathepsin L inactivates alpha 1-proteinase inhibitor by cleavage in the reactive site region. J Biol Chem. 1986 Nov 5;261(31):14748–14751. [PubMed] [Google Scholar]
- Joseph L., Lapid S., Sukhatme V. The major ras induced protein in NIH3T3 cells is cathepsin L. Nucleic Acids Res. 1987 Apr 10;15(7):3186–3186. doi: 10.1093/nar/15.7.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis I. G., Drenth J., Baker E. N. Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain. J Mol Biol. 1985 Mar 20;182(2):317–329. doi: 10.1016/0022-2836(85)90348-1. [DOI] [PubMed] [Google Scholar]
- Kavathas P., Sukhatme V. P., Herzenberg L. A., Parnes J. R. Isolation of the gene encoding the human T-lymphocyte differentiation antigen Leu-2 (T8) by gene transfer and cDNA subtraction. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7688–7692. doi: 10.1073/pnas.81.24.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H., Kembhavi A. A., Bohley P., Barrett A. J. Action of rat liver cathepsin L on collagen and other substrates. Biochem J. 1982 Feb 1;201(2):367–372. doi: 10.1042/bj2010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominami E., Kunio I., Katunuma N. Activation of the intramyofibral autophagic-lysosomal system in muscular dystrophy. Am J Pathol. 1987 Jun;127(3):461–466. [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes in normal and disease states. J Clin Invest. 1986 Jan;77(1):1–6. doi: 10.1172/JCI112262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lemansky P., Hasilik A., von Figura K., Helmy S., Fishman J., Fine R. E., Kedersha N. L., Rome L. H. Lysosomal enzyme precursors in coated vesicles derived from the exocytic and endocytic pathways. J Cell Biol. 1987 Jun;104(6):1743–1748. doi: 10.1083/jcb.104.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Johnson D. A., Barrett A. J., Chapman H. A. Elastinolytic activity of human cathepsin L. Biochem J. 1986 Feb 1;233(3):925–927. doi: 10.1042/bj2330925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Walker J. E., Northrop F. D. The N-terminal amino acid sequences of the heavy and light chains of human cathepsin L. Relationship to a cDNA clone for a major cysteine proteinase from a mouse macrophage cell line. Biochem J. 1986 Dec 1;240(2):373–377. doi: 10.1042/bj2400373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer R. J., Doherty F. Intracellular protein catabolism: state of the art. FEBS Lett. 1986 Mar 31;198(2):181–193. doi: 10.1016/0014-5793(86)80403-3. [DOI] [PubMed] [Google Scholar]
- Muschel R. J., Williams J. E., Lowy D. R., Liotta L. A. Harvey ras induction of metastatic potential depends upon oncogene activation and the type of recipient cell. Am J Pathol. 1985 Oct;121(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbricht C. J., Cannon J. K., Garg L. C., Tisher C. C. Activities of cathepsins B and L in isolated nephron segments from proteinuric and nonproteinuric rats. Am J Physiol. 1986 Jun;250(6 Pt 2):F1055–F1062. doi: 10.1152/ajprenal.1986.250.6.F1055. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Erickson A. H., Kochan J., Ravetch J. V., Unkeless J. C. Cloning and characterization of a mouse cysteine proteinase. J Biol Chem. 1986 Nov 5;261(31):14697–14703. [PubMed] [Google Scholar]
- Pozzatti R., Muschel R., Williams J., Padmanabhan R., Howard B., Liotta L., Khoury G. Primary rat embryo cells transformed by one or two oncogenes show different metastatic potentials. Science. 1986 Apr 11;232(4747):223–227. doi: 10.1126/science.3456644. [DOI] [PubMed] [Google Scholar]
- Rabin M. S., Doherty P. J., Gottesman M. M. The tumor promoter phorbol 12-myristate 13-acetate induces a program of altered gene expression similar to that induced by platelet-derived growth factor and transforming oncogenes. Proc Natl Acad Sci U S A. 1986 Jan;83(2):357–360. doi: 10.1073/pnas.83.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recklies A. D., Mort J. S. Rat mammary gland in culture secretes a stable high molecular weight form of cathepsin L. Biochem Biophys Res Commun. 1985 Aug 30;131(1):402–407. doi: 10.1016/0006-291x(85)91816-9. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E., Tronick S. R., Aaronson S. A., Pulciani S., Barbacid M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature. 1982 Jul 22;298(5872):343–347. doi: 10.1038/298343a0. [DOI] [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- St John T. P., Davis R. W. Isolation of galactose-inducible DNA sequences from Saccharomyces cerevisiae by differential plaque filter hybridization. Cell. 1979 Feb;16(2):443–452. doi: 10.1016/0092-8674(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Sugano S., Stoeckle M. Y., Hanafusa H. Transformation by Rous sarcoma virus induces a novel gene with homology to a mitogenic platelet protein. Cell. 1987 May 8;49(3):321–328. doi: 10.1016/0092-8674(87)90284-4. [DOI] [PubMed] [Google Scholar]
- Takio K., Towatari T., Katunuma N., Teller D. C., Titani K. Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3666–3670. doi: 10.1073/pnas.80.12.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida K., Aihara H., Isogai K., Hanada K., Shibata N. Degradation of myocardial structural proteins in myocardial infarcted dogs is reduced by Ep459, a cysteine proteinase inhibitor. Biol Chem Hoppe Seyler. 1986 Jan;367(1):39–45. doi: 10.1515/bchm3.1986.367.1.39. [DOI] [PubMed] [Google Scholar]
- Viola M. V., Fromowitz F., Oravez S., Deb S., Finkel G., Lundy J., Hand P., Thor A., Schlom J. Expression of ras oncogene p21 in prostate cancer. N Engl J Med. 1986 Jan 16;314(3):133–137. doi: 10.1056/NEJM198601163140301. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]