Abstract
Purpose
We compared the resistance patterns of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible Staphylococcus aureus (MSSA) keratitis isolates to common topically applied ophthalmic antimicrobials.
Methods
We reviewed the antibiotic susceptibility results of 122 MRSA and 276 MSSA keratitis isolates from January 1993 to November 2012. In vitro susceptibility testing of each SA isolate was performed using Kirby-Bauer disk diffusion based on modified serum interpretations for cefoxitin, bacitracin, cefazolin, ciprofloxacin, gatifloxacin, gentamicin, moxifloxacin, ofloxacin, polymyxin B, sulfamethoxazole, tobramycin, and trimethoprim.
Results
MRSA represented 30.7% (122 of 398) of the total SA isolates. All SA isolates were susceptible to vancomycin, while less susceptible to the fluoroquinolones than to the non-fluoroquinolones. In comparison to MSSA, MRSA was significantly more resistant to all antibiotics tested other than polymyxin B (both equally resistant) and vancomycin (both equally susceptible) (p<0.001). Besides vancomycin, MRSA demonstrated the best susceptibilities to sulfamethoxazole (94.3%), bacitracin (89.3%), trimethoprim (88.5%), and gentamicin (86.1%). Additionally, MRSA was found to be significantly more resistant to the second-generation fluoroquinolones (ciprofloxacin and ofloxacin) than to the fourth-generation fluoroquinolones (moxifloxacin and gatifloxacin). An increase in resistance to the fourth-generation fluoroquinolones was detected for both MRSA and MSSA over the study period.
Conclusions
The in vitro susceptibilities of commonly used topical antibiotics differ for MRSA and MSSA isolates, thus successful treatment of bacterial keratitis should be supported with laboratory studies. Vancomycin remains the treatment of choice for MRSA keratitis. The empiric use of second-generation fluoroquinolones appears to be contraindicated in the treatment of MRSA keratitis.
Keywords: Staphylococcus keratitis, MRSA, MSSA, antimicrobials
INTRODUCTION
Staphylococcus aureus (SA) is a leading cause of keratitis worldwide.1,2 SA is considered the most virulent of all the Staphylococcus species, possessing a multitude of factors that enhance host-adhesion, evasion of the human innate immune system, and cytolytic activity against host cells.3,4 Approximately one-third of the population is colonized with SA, which increases the risk for associated ocular infections.5,6 Violation of the epithelial barrier such as with contact lens use or other trauma can result in subsequent corneal ulceration, necessitating aggressive treatment with topical antibiotics.5,7
SA has emerged as a major public-health threat due to the organism’s propensity to develop resistances against antibiotics. Historically, SA developed resistance to Penicillin G within two years of its introduction in 1942.5 Methicillin was introduced in 1959 to combat the emergence of penicillinase containing SA; however, methicillin-resistant SA (MRSA) was reported just one year later.6 The first case of SA with reduced susceptibilities to vancomycin was reported in 1997, and has become a more recent concern in the treatment of SA infections.8 Fortunately, reports of complete vancomycin resistance continue to remain rare.6
By practical definition, MRSA is resistant to all beta-lactam antibiotics, including oxacillin, nafcillin, dicloxacillin, and cefazolin, through production of beta-lactamases, mutation of the normal penicillin binding protein, and/or acquisition of the mecA gene that encodes for an alternative penicillin-binding protein.5,9,10 The increase in MRSA ocular infections resulting in devastating consequences such as corneal perforations, flap melts after refractive surgery, cellulitis, and endophthalmitis has been published.9,11–17 Although these reports are troublesome, studies have suggested the majority of MRSA ocular manifestations tend not to be visually devastating.9,12
Empiric therapy is often initiated prior to the return of corneal smears and cultures in the treatment of bacterial keratitis. For ulcers less than 2mm, practitioners may not routinely perform cultures prior to starting a broad-spectrum antibiotic.18 Since microbial resistance patterns can vary by year and geographical region, local annual surveys are important in guiding the empiric treatment of bacterial keratitis.
The purpose of this analysis was to determine the prevalence, distribution, and in vitro resistance patterns of MRSA keratitis isolates compared to methicillin-susceptible SA (MSSA) keratitis isolates to commonly used ophthalmic antimicrobials in a twenty-year retrospective review. We hypothesize that MRSA and MSSA will differ in their resistance patterns, and MRSA will have significantly increased resistance to all tested antibiotics in comparison to MSSA with the exception of vancomycin.
MATERIALS AND METHODS
The incidence of bacterial keratitis and the laboratory antibiotic susceptibility patterns of SA keratitis isolates presenting to the Charles T. Campbell Ophthalmic Microbiology Laboratory at the University of Pittsburgh Medical Center (UPMC) over twenty years (January 2, 1993-November 26, 2012) were reviewed and analyzed. This laboratory data was de-identified and not from the patients’ medical records. The data was used for the calculation of antibiotic susceptibility patterns, which is mandatory for laboratory certification. The laboratory data was reviewed consecutively in reverse chronological order from November 26, 2012 without the use of patient identifiers (University of Pittsburgh, exemption IRB # PRO14030138). Cultures positive for SA were defined as significant growth collected from the cornea. Patients with growth collected only from the conjunctiva and/or eyelid were not included in this study.
Methicillin resistance was determined originally with oxacillin and later with cefoxitin using the Kirby-Bauer disk diffusion method (National Committee for Clinical Laboratory and Standards Institute (CLSI), Wayne, PA).19 The identification of MRSA using cefoxitin is thought to allow for more sensitive detection of mecA-mediated resistance as compared to oxacillin.20 In vitro laboratory susceptibility testing of each SA isolate was also performed using the Kirby-Bauer disk diffusion method to bacitracin, cefazolin, ciprofloxacin, gatifloxacin, gentamicin, moxifloxacin, ofloxacin, polymyxin B, sulfamethoxazole, tobramycin, and trimethoprim.19 Isolates identified as intermediate susceptibility were considered susceptible to all antibiotics with the exception of cefoxitin and polymyxin B, for which isolates of intermediate susceptibility were grouped with the resistant isolates. Isolates with intermediate susceptibility to Cefoxitin may indicate the presence of the mecA gene, and thus were best classified as Cefoxitin resistant.18–20 Polymyxin B is considered a Gram-negative antibiotic that does not diffuse well in medium, and resistance to this antibiotic is characteristic of Staphylococcus aureus.18–20 Thus, intermediate susceptible isolates were categorized as polymyxin B resistant.
Besifloxacin was not tested for in vitro susceptibility because there is a lack of a susceptibility standard and commercial source for disks and powder. Cefazolin susceptibility was tested since the literature does demonstrate MRSA susceptibility to cephalosporin antibiotics.21–24
It must be noted that there are no susceptibility standards for topical therapy. The serum standards can be used with the assumption that antibiotic concentrations in the ocular tissue are equal or greater than the concentration of antibiotics in the serum. This likely allows for the over-reporting of resistance.
All statistical analysis of in vitro susceptibility results of MSSA and MRSA was performed using chi-square (MiniTab, State College, PA and SPSS, IBM Corp. 2011, Version 20, Armonk, NY). Differences between SA resistance rates between the first and second decades of the study were determined by the Fisher’s exact test (SPSS). A p-value of 0.05 or less was considered to be statistically significant for all analysis performed.
RESULTS
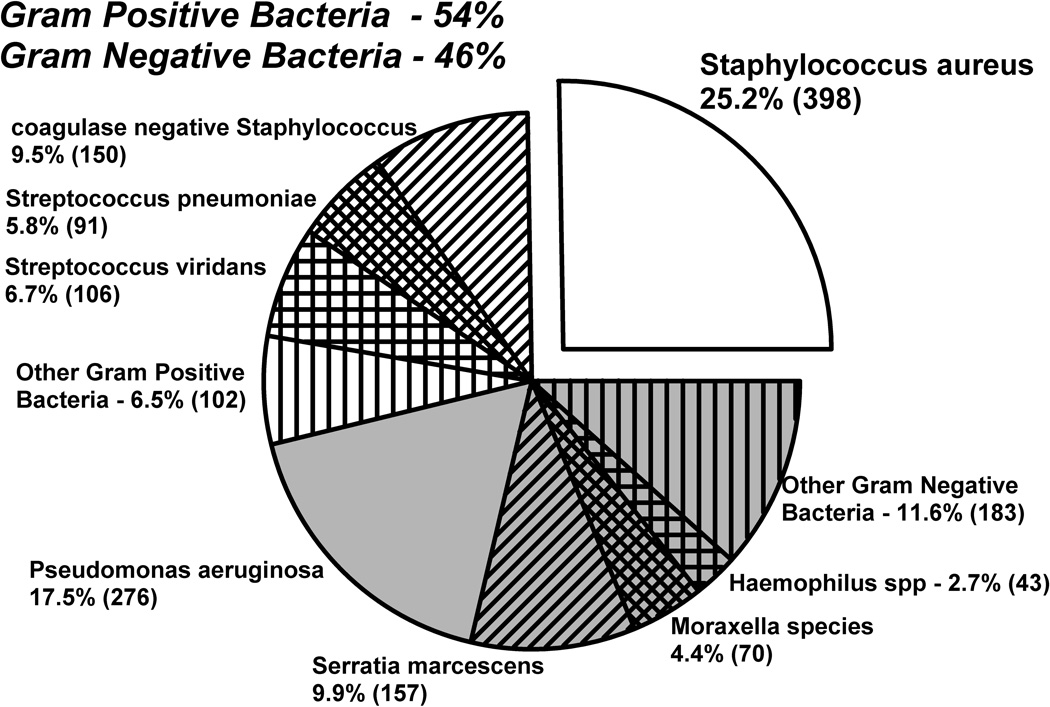
Figure 1 illustrates the distribution of bacterial keratitis from January 1993 to November 2012 (N=1576). Overall, there were more Gram-positive organisms (54%, 869 out of 1576 isolates) than Gram-negative organisms (46%, 729 out of 1576 isolates). However, there was not a statistically significant difference between the incidences of Gram-negative and Gram-positive infections (p= 0.346, chi-square). SA (25.2%) was found to be the most common cause of bacterial keratitis.
FIGURE 1.
The distribution of 1576 bacterial keratitis isolates from January 1993 to November 2012.
More SA isolates were susceptible to the non-fluoroquinolones compared to the fluoroquinolones (p<0.05, chi-square). In addition, more SA isolates were resistant to tobramycin (95 out of 398 SA isolates) compared to gentamicin (23 out of 398 SA isolates) (p=0.0001, chi-square); whereas, susceptibilities to bacitracin (95.2%), gentamicin (94.2%), sulfamethoxazole (97.4%), and trimethoprim (95.7%) were statistically equivalent (p=0.176, chi-square). All SA isolates were susceptible to vancomycin.
The 398 SA isolates were further subdivided into two categories: MSSA and MRSA. There were a total of 122 MRSA isolates that represented 30.7% (122 out of 398) of the total SA isolates and 7.7% (122 of 1576) of the total bacterial isolates.
Table 1 lists the prevalence of MRSA in four-year intervals from 1993 to 2012. The ratio of MSSA to MRSA changed from greater than 4:1 to less than 2:1 from the first four years (1993 to 1996) to the last four years (2009 to 2012) of the study. There was a statistically significant increase in the prevalence of MRSA keratitis over time (p= 0.001, chi-square).
TABLE 1.
Prevalence of MSSA and MRSA in 4-year intervals over 20 years.
| 1993–1996 | 1997–2000 | 2001–2004 | 2005–2008 | 2009–2012 | |
|---|---|---|---|---|---|
| MSSA | 93 (81.2%) | 50 (69.4%) | 60 (65.2%) | 36 (55.4%) | 37 (61.7%) |
| MRSA | 21 (18.4%) | 22 (30.6%) | 32 (34.8%) | 24 (44.6%) | 23 (38.3%) |
| TOTAL | 114 | 72 | 92 | 60 | 60 |
The overall resistances of MRSA and MSSA to the aforementioned antibiotics during the study period are listed in Table 2. As shown, a significantly greater number of MRSA isolates was found to be resistant to all antibiotics tested compared to MSSA (p<0.05, chi-square) with the exclusion of polymyxin B (resistant to all) and vancomycin (susceptible to all).
TABLE 2.
Resistance rates of MSSA and MRSA to commonly used topical ophthalmic antibiotics from 1993–2012.
| ANTIBIOTIC | MSSA | MRSA | p-value (chi-square) |
|---|---|---|---|
| Bacitracin | 2.2% | 10.7% | 0.0001 |
| Cefazolin | 0.7% | 33.6% | 0.0001 |
| Gentamicin | 2.2% | 13.9% | 0.0001 |
| Polymyxin B | 97.5% | 100.0% | 0.076 |
| Sulfamethoxazole | 1.1% | 5.7% | 0.008 |
| Tobramycin | 7.6% | 60.7% | 0.0001 |
| Trimethoprim | 1.1% | 11.5% | 0.0001 |
| Ciprofloxacin | 10.1% | 73.8% | 0.0001 |
| Ofloxacin | 11.2% | 74.6% | 0.0001 |
| Moxifloxacin | 5.8% | 35.2% | 0.0001 |
| Gatifloxacin | 8.3% | 45.9% | 0.0001 |
| Vancomycin | 0.0% | 0.0% | -- |
MSSA
MSSA retained better than 90% susceptibility to all antibiotics tested over the study period. There was no significant difference between percent susceptibilities to bacitracin (97.8%), cefazolin (99.3%), gentamicin (97.8%), sulfamethoxazole (98.9%), and trimethoprim (98.9%) (p=0.137, chi-square). A significantly greater number of MSSA isolates was found to be resistant to the fluoroquinolones compared to the non-fluoroquinolones (p<0.05, chi-square); however, no difference was detected between the second and fourth-generation fluoroquinolones (p= 0.173, chi-square). There was a significant increase in resistance from the first to the second decade of the study period for ofloxacin (p=0.003, Fisher’s exact test), gatifloxacin (p=0.044, Fisher’s exact test), and moxifloxacin (p=0.001, Fisher’s exact test).
MRSA
Besides vancomycin, MRSA retained the best susceptibilities to sulfamethoxazole (94.3%), bacitracin (89.3%), trimethoprim (88.5%), and gentamicin (86.1%). No significant difference between percent resistances of MRSA to these four antibiotics was detected (p>0.05, chi-square). There was greater resistance against the fluoroquinolones compared to the non-fluoroquinolones (p< 0.05, chi-square). Unlike MSSA, MRSA was found to be significantly more resistant to the second-generation compared to the fourth-generation fluoroquinolones (p= 0.0001, chi-square). A significant increase in resistance between the first and second decades of the study for moxifloxacin (p=0.022, Fisher’s exact test) and gatifloxacin (p=0.045, Fisher’s exact test) was detected.
DISCUSSION
Whether or not methicillin-resistance confers greater virulence to SA is a topic of hot debate in the general medical literature.6,25–27 Community-acquired MRSA (CA-MRSA) can produce Panton-Valentine leukocidin (PVL), alpha-toxin, and phenol-soluble modulins (PSMs), which may exacerbate ocular inflammation.1,2,6,28 However, reports suggest the majority of ocular MRSA infections are equivocal to MSSA in terms of complication rates and final visual outcomes.3,4,11,20,29,30 Furthermore, the clinical virulence of MRSA is likely overrepresented in the literature since only the more serious infections are cultured and reported.5,6,12,25,26
Vancomycin is widely regarded as the gold-standard in the treatment of MRSA keratitis.5,7,12,18,20,26,31 However, as a fortified antibiotic, vancomycin is far from ideal given its cost, toxicity, short half-life, and the need for refrigeration. Additionally, vancomycin is considered less bactericidal in comparison to the beta-lactams, which may have important clinical implications in regards to the emergence of MRSA with reduced glycopeptide susceptibilities.5,32 Although the risk of systemic resistance from topical antimicrobials is likely minimal, increased extraocular resistance has been reported with the use of topical tetracycline in the treatment of trachoma.6,33 Fortunately, no culture-proven vancomycin-resistant SA (VRSA) has been reported in the ophthalmic literature thus far.8,34
In our review, the prevalence of MRSA keratitis increased during the study period, consistent with reports worldwide.2,6,9,17,35 MRSA retained better in vitro susceptibilities to sulfamethoxazole, bacitracin, gentamicin, and trimethoprim in comparison to the fluoroquinolones. Surprisingly, MRSA had lower susceptibility to gentamicin than anticipated (85.94%), as a survey published at the same institution from 1993 to 2010 showed 100% susceptibility to gentamicin for nine MRSA isolates.,18 In a review of the literature, ocular MRSA susceptibility rates tended to vary; although, susceptibility to sulfamethoxazole/trimethoprim was generally reported to be high (greater than 90%), which was consistent with our findings.5,9,11–17,29 However, some studies reported lower MRSA susceptibility rates to sulfamethoxazole/trimethoprim, ranging from 66.6% to 80%. 9,11,12,16,20,36 We believe these differences reflect regional and populational variations, and recognize that our results may differ from other studies.
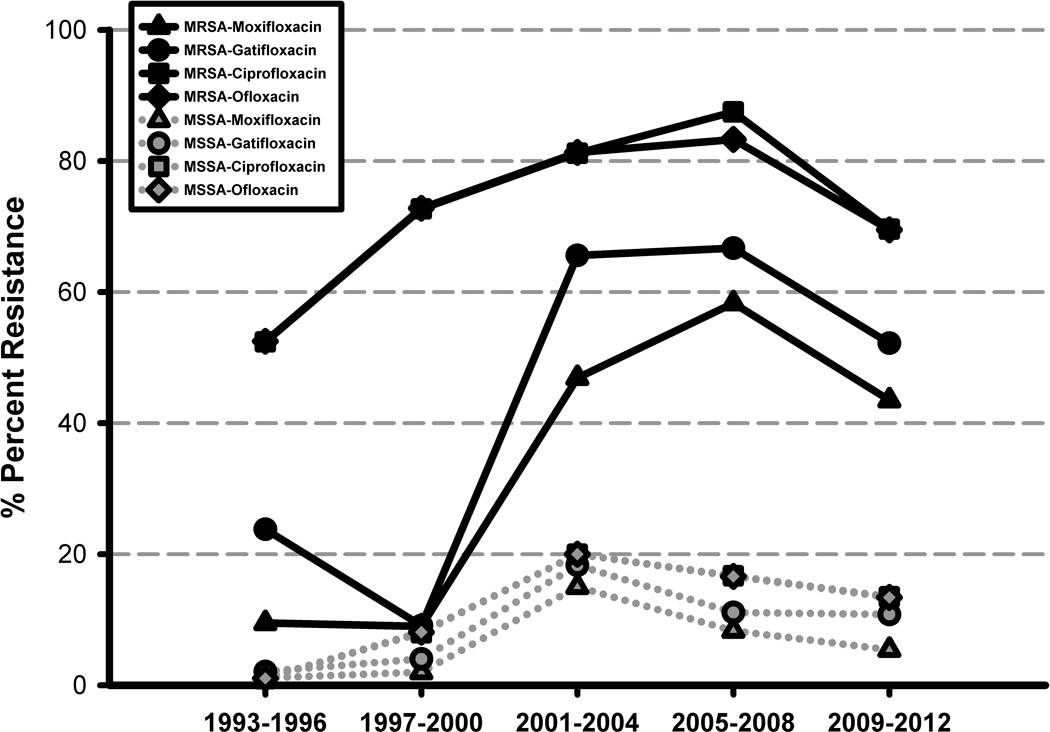
Increased microbial resistance to fluoroquinolones, the most commonly used empiric monotherapy in the treatment of bacterial keratitis, has been widely reported.1,18,20,31,37–39 In our review, the overall susceptibilities to the fluoroquinolones were poor. Figure 2 demonstrates increasing trends in MSSA and MRSA resistances to fluoroquinolones during the study period. A greater number of MRSA isolates, as seen in figure 2, were resistant to the fluoroquinolones compared to MSSA (p=0.001, chi-square). For MRSA, overall resistance rates remained higher against the “older” fluoroquinolones (ciprofloxacin and ofloxacin) compared to the “newer” fluoroquinolones (moxifloxacin and gatifloxacin). Second-generation fluoroquinolones are smaller, more hydrophobic, and less soluble, allowing for easier efflux out of the microorganism.35 Furthermore, only one mutation (against gyrase) is needed for resistance against the second-generation fluoroquinolones, whereas two mutations (against gyrase and topoisomerase IV) are needed to confer resistance against fourth-generation fluoroquinolones.18,40 Interestingly, an increase in the resistance rates during the study period was found for the fourth-generation fluoroquinolones for both MSSA and MRSA (p<0.05, Fisher’s exact test). Prior use of topical fluoroquinolones has been associated with increased in vitro resistance.41 These results likely reflect on the increasing popularity of moxifloxacin and gatifloxacin, since their introduction in 2003, in the empiric treatment of bacterial keratitis.37,39 We postulate that antibiotics such as trimethoprim, sulfamethoxazole, bacitracin, and gentamicin possess better susceptibility profiles since they remain less popular compared to the fluoroquinolones.
FIGURE 2.
MSSA and MRSA: Increasing resistance against the fluoroquinolones over 20 years.
Low levels of in vitro resistance against an anti-infective do not necessarily correlate with poor clinical efficacy. Antibiotic concentrations are much higher in ocular tissue via topical therapy in comparison to systemic therapy.42 Since the susceptibility and resistance patterns of the aforementioned antibiotics were determined using systemic standards, there is likely an over-estimation of laboratory resistance rates. Furthermore, in vitro studies do not take into account pharmacokinetics, such as the excellent corneal penetration of fourth-generation fluoroquinolones.43
Additional limitations include that as an in vitro study, dosing, local immunity, and patient compliance are not taken into account. However, in vitro studies are considered the standard in determining antibiotic resistances. With the introduction of broad-spectrum antibiotics such as the fluoroquinolones, community ophthalmologists are more comfortable empirically treating smaller ulcers without laboratory support. This likely contributed to the decline in the number of corneal cultures sent to UPMC, a tertiary referral center, through the years. Thus, a selection bias for the larger and more clinically aggressive isolates may have resulted. Despite this, we believe our results, which showed increasing MRSA prevalence and antimicrobial resistance are comparable with the worldwide literature. Further studies are needed, however, to determine the clinical relevance of these laboratory findings.
In conclusion, empiric antimicrobial selection should be guided by annual regional surveillance surveys, and therapy should be optimized by laboratory susceptibilities and clinical response in the treatment of bacterial keratitis. Our data supports the hypothesis that MRSA and MSSA differ in their resistance patterns. MRSA isolates have significantly increased resistance to all tested antibiotics in comparison to MSSA, with the exception of polymyxin B and vancomycin. Finally, although vancomycin is our first-line choice of treatment in cases of MRSA keratitis, our results suggest other commonly used topical antibiotics such as sulfamethoxazole, trimethoprim, gentamicin, and bacitracin ointment may be adjunctively considered with the guidance of laboratory support. The empiric use of second-generation fluoroquinolone anti-infectives in the treatment of MRSA keratitis should be avoided.
ACKNOWLEDGEMENTS
Statistical analysis was performed in part by the University of Pittsburgh’s Clinical and Translational Science Institute with the support of the NIH through Grant Number UL1TR000005.
Support: This work was supported by The Eye and Ear Foundation of Pittsburgh, Pittsburgh, Pennsylvania; an NIH core grant for vision research P30 EY008098; and, an unrestricted grant for research to prevent blindness, Inc., New York, New York.
Footnotes
Disclosure: The authors have no proprietary interest in the products discussed in this article. No conflicting relationship exists for any author.
Presented in part at the Association for Research in Vision and Ophthalmology annual meeting, Orlando, Florida, May 2014.
REFERENCES
- 1.Orlans HO, Hornby SJ, Bowler ICJW. In vitro antibiotic susceptibility patterns of bacterial keratitis isolates in Oxford, UK:a 10-year review. Eye (Lond) 2011;25:489–493. doi: 10.1038/eye.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marangon FB, Miller D, Muallem MS, et al. Ciprofloxacin and levofloxacin resistance among methicillin-sensitive staphylococcus aureus isolates from keratitis and conjunctivitis. Am J Ophthalmol. 2004;137:453–458. doi: 10.1016/j.ajo.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Zecconi A, Scali F. Staphylococcus aureus virulence factors in evasion from innate immune defenses in human and animal diseases. Immunol Lett. 2013;150:12–22. doi: 10.1016/j.imlet.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Hiramatsu K, Ito T, Tsubakishita S, et al. Genomic Basis for Methicillin Resistance in Staphylococcus aureus. Infect Chemother. 2013;45:117–136. doi: 10.3947/ic.2013.45.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blomquist PH. Methicillin-resistant Staphylococcus aureus infections of the eye and orbit (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:322–345. [PMC free article] [PubMed] [Google Scholar]
- 6.Otto M. Community-associated MRSA: What makes them special? Int J Med Microbiol. 2013;303:324–330. doi: 10.1016/j.ijmm.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi S, Suzuki T, Yamaguchi S, et al. Genotypic characterization of Staphylococcus aureus isolates from cases of keratitis and healthy conjunctival sacs. Cornea. 2014;33:72–76. doi: 10.1097/ICO.0b013e3182a4810f. [DOI] [PubMed] [Google Scholar]
- 8.Fridkin SK, Hageman J, McDougal LK, et al. Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997–2001. Clin Infect Dis. 2003;36:429–439. doi: 10.1086/346207. [DOI] [PubMed] [Google Scholar]
- 9.Vola ME, Moriyama AS, Lisboa R, et al. Prevalence and antibiotic susceptibility of methicillin-resistant Staphylococcus aureus in ocular infections. Arq Bras Oftalmol. 2013;76:350–353. doi: 10.1590/s0004-27492013000600006. [DOI] [PubMed] [Google Scholar]
- 10.Xia J, Gao J, Kokudo N, et al. Methicillin-resistant Staphylococcus aureus antibiotic resistance and virulence. Biosci Trends. 2013;7:113–121. [PubMed] [Google Scholar]
- 11.Ong SJ, Huang Y-C, Tan H-Y, et al. Staphylococcus aureus Keratitis: A Review of Hospital Cases. PLoS ONE. 2013;8:e80119. doi: 10.1371/journal.pone.0080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freidlin J, Acharya N, Lietman TM, et al. Spectrum of eye disease caused by methicillin-resistant Staphylococcus aureus. Am J Ophthalmol. 2007;144:313–315. doi: 10.1016/j.ajo.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 13.Magli A, Forte R, Rombetto L, et al. Bilateral methicillin-resistant Staphylococcus aureus keratitis following hyperopic photorefractive surgery. Int Ophthalmol. 2012;32:47–49. doi: 10.1007/s10792-011-9505-1. [DOI] [PubMed] [Google Scholar]
- 14.Solomon R, Donnenfeld ED, Holland EJ, et al. Microbial keratitis trends following refractive surgery: Results of the ASCRS infectious keratitis survey and comparisons with prior ASCRS surveys of infectious keratitis following keratorefractive procedures. J Cataract Refract Surg. 2011;37:1343–1350. doi: 10.1016/j.jcrs.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Zhou AW, Lee MC, Rudnisky CJ. Ocular microbiology trends in Edmonton, Alberta: a 10-year review. Can J Ophthalmol. 2012;47:301–304. doi: 10.1016/j.jcjo.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Lichtinger A, Yeung SN, Kim P, et al. Shifting Trends in Bacterial Keratitis in Toronto. Ophthalmology. 2012;119:1785–1790. doi: 10.1016/j.ophtha.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 17.Sotozono C, Inagaki K, Fujita A, et al. Methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis infections in the cornea. Cornea. 2002;21:S94–S101. doi: 10.1097/01.ico.0000263127.84015.3f. [DOI] [PubMed] [Google Scholar]
- 18.Kowalski RP, Kowalski TA, Shanks RMQ, et al. In vitro comparison of combination and monotherapy for the empiric and optimal coverage of bacterial keratitis based on incidence of infection. Cornea. 2013;32:830–834. doi: 10.1097/ICO.0b013e318268d6f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard. 8th ed. Vol. 29. Wayne, PA: Clinical and Laboratory Standards Institute; 2009. [Google Scholar]
- 20.Chuang C-C, Hsiao C-H, Tan H-Y, et al. Staphylococcus aureus Ocular Infection: Methicillin-Resistance, Clinical Features, and Antibiotic Susceptibilities. PLoS ONE. 2012;8:e42437. doi: 10.1371/journal.pone.0042437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chambers HF, Sachdeva M. Binding of beta-lactam antibiotics to penicillin-binding proteins in methicillin-resistant Staphylococcus aureus. J Infect Dis. 1990;161:1170–1176. doi: 10.1093/infdis/161.6.1170. [DOI] [PubMed] [Google Scholar]
- 22.Hikida M, Mori M, Yoshida M, et al. Possible usefulness of cephem antibiotics with anti-staphylococcal activity for preventing the spread of methicillin-resistant Staphylococcus aureus. Jpn J Antibiot. 1993;46:836–839. [PubMed] [Google Scholar]
- 23.Entenza JM, Hohl P, Heinze-Krauss I, et al. BAL9141, a Novel Extended-Spectrum Cephalosporin Active against Methicillin-Resistant Staphylococcus aureus in Treatment of Experimental Endocarditis. Antimicrob Agents Chemother. 2002;46:171–177. doi: 10.1128/AAC.46.1.171-177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fallon MT, Shafer W, Jacob E. Use of cefazolin microspheres to treat localized methicillin-resistant Staphylococcus aureus infections in rats. J Surg Res. 1999;86:97–102. doi: 10.1006/jsre.1999.5686. [DOI] [PubMed] [Google Scholar]
- 25.Cosgrove SE, Qi Y, Kaye KS, et al. The Impact of Methicillin Resistance in Staphylococcus aureus Bacteremia on Patient Outcomes: Mortality, Length of Stay, and Hospital Charges. Infect Control Hosp Epidemiol. 2005;26:166–174. doi: 10.1086/502522. [DOI] [PubMed] [Google Scholar]
- 26.Harbarth S, Rutschmann O, Sudre P, et al. Impact of methicillin resistance on the outcome of patients with bacteremia caused by Staphylococcus aureus. Arch Intern Med. 1998;158:182–189. doi: 10.1001/archinte.158.2.182. [DOI] [PubMed] [Google Scholar]
- 27.Blot SI, Vandewoude KH, Hoste EA, et al. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med. 2002;162:2229–2235. doi: 10.1001/archinte.162.19.2229. [DOI] [PubMed] [Google Scholar]
- 28.Zaidi T, Zaidi T, Yoong P, et al. Staphylococcus aureus corneal infections: effect of the Panton-Valentine leukocidin (PVL) and antibody to PVL on virulence and pathology. Invest Ophthalmol Vis Sci. 2013;54:4430–4438. doi: 10.1167/iovs.13-11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amato M, Pershing S, Walvick M, et al. Trends in ophthalmic manifestations of methicillin-resistant Staphylococcus aureus (MRSA) in a northern California pediatric population. J AAPOS. 2013;17:243–247. doi: 10.1016/j.jaapos.2012.12.151. [DOI] [PubMed] [Google Scholar]
- 30.Shanmuganathan VA, Armstrong M, Buller A, et al. External ocular infections due to methicillin-resistant Staphylococcus aureus (MRSA) Eye (Lond) 2004;19:284–291. doi: 10.1038/sj.eye.6701465. [DOI] [PubMed] [Google Scholar]
- 31.Hsiao CH, Chuang CC, Tan HY, et al. Methicillin-Resistant Staphylococcus aureus Ocular Infection: A 10-Year Hospital-Based Study. Ophthalmology. 2012;119:522–527. doi: 10.1016/j.ophtha.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 32.Gould IM. Treatment of bacteraemia: Methicillin-resistant Staphylococcus aureus (MRSA) to vancomycin-resistant S. aureus (VRSA) Int J Antimicrob Agents. 2013;42:S17–S21. doi: 10.1016/j.ijantimicag.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Gaynor BD. Topical ocular antibiotics induce bacterial resistance at extraocular sites. Br J Ophthalmol. 2005;89:1097–1099. doi: 10.1136/bjo.2005.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawser SP, Bouchillon SK, Hoban DJ, et al. Rising incidence of Staphylococcus aureus with reduced susceptibility to vancomycin and susceptibility to antibiotics: a global analysis 2004–2009. Int J Antimicrob Agents. 2011;37:219–224. doi: 10.1016/j.ijantimicag.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 35.Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: Nationwide Antimicrobial Susceptibility Patterns in Ocular Isolates. Am J Ophthalmol. 2008;145:951.e1–958.e1. doi: 10.1016/j.ajo.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 36.Elsahn AF, Yildiz EH, Jungkind DL, et al. In Vitro Susceptibility Patterns of Methicillin-Resistant Staphylococcus aureus and Coagulase-Negative Staphylococcus Corneal Isolates to Antibiotics. Cornea. 2010;29:1131–1135. doi: 10.1097/ICO.0b013e3181d2ce25. [DOI] [PubMed] [Google Scholar]
- 37.Afshari NA, Ma JJK, Duncan SM, et al. Trends in Resistance to Ciprofloxacin, Cefazolin, and Gentamicin in the Treatment of Bacterial Keratitis. J Ocul Pharmacol Ther. 2008;24:217–223. doi: 10.1089/jop.2007.0085. [DOI] [PubMed] [Google Scholar]
- 38.Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in South Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107:1497–1502. doi: 10.1016/s0161-6420(00)00179-2. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis. Ophthalmology. 1999;106:1213–1318. [PubMed] [Google Scholar]
- 40.Oliveira ADD, d'Azevedo PA, Francisco W, et al. In vitro activity of fluoroquinolones against ocular bacterial isolates in Sao Paulo, Brazil. Cornea. 2007;26:194–198. doi: 10.1097/01.ico.0000248379.78777.f6. [DOI] [PubMed] [Google Scholar]
- 41.Fintelmann RE, Hoskins EN, Lietman TM, et al. Topical fluoroquinolone use as a risk factor for in vitro fluoroquinolone resistance in ocular cultures. Arch Ophthalmol. 2011;129:399–402. doi: 10.1001/archophthalmol.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowalski RP. Is antibiotic resistance a problem in the treatment of ophthalmic infections? Expert Rev Ophthal. 2013;8:119–126. [Google Scholar]
- 43.Romanowski EG, Mah FS, Yates KA, et al. The Successful Treatment of Gatifloxacin-resistant Staphylococcus aureus Keratitis With Zymar® (Gatifloxacin 0.3%) in a NZW Rabbit Model. Am J Ophthalmol. 2005;139:867–877. doi: 10.1016/j.ajo.2005.01.021. [DOI] [PubMed] [Google Scholar]