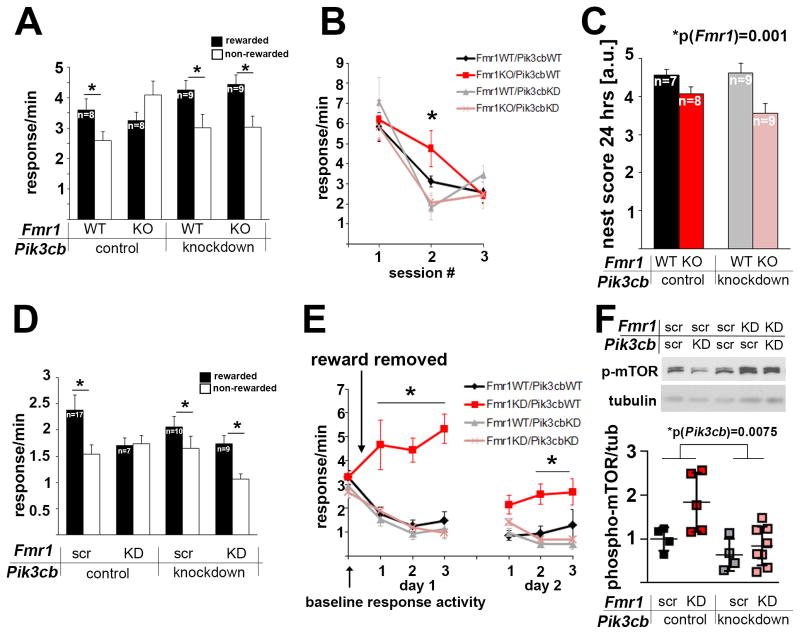
Figure 1. PFC-selective reduction of p110β restores goal-directed decision-making and behavioral flexibility in Fmr1KO and PFC-selective Fmr1 knockdown mice.
(A) Fmr1KO mice were unable to differentiate between rewarded and non-rewarded actions after extended training and reversal of the response-outcome contingencies, although they were initially able to acquire the task (see Figures S1A and B). This deficiency was rescued by PFC-selective Pik3cb knockdown (3-way ANOVA, p(Fmr1 × Pik3cb × aperture interaction)=0.023, F(1,30)=5.7; post-hoc comparisons p<0.05 in all groups except for the Fmr1KO mice). Also see Figure S1C showing reduction of p110β protein in PFC tissue punches following lentiviral shRNA knockdown, and Figure S1D showing restriction of virus expression to the PFC.
(B) Mice were trained to press a lever for a food reinforcer, leading to similar response rates in all four groups. When the food was withheld, Fmr1KO mice showed delayed extinction, which was reversed by concurrent PFC-selective Pik3cb knockdown (3-way ANOVA, p(Fmr1 × Pik3cb × session interaction)=0.03, F(1,27)=5, *p<0.05).
(C) Impaired nesting behavior in Fmr1KO mice was not rescued by PFC-selective Pik3cb knockdown (2-way ANOVA, p(Fmr1)=0.001, F(1,29)=12.7; p(Pik3cb)=0.32, F(1,29)=1.0; p(interaction)=0.206, F(1,29)=1.7).
(D) Mice with PFC-selective knockdown of Fmr1 were unable to differentiate between trained actions that were or were not rewarded after reversal of action-outcome relationship, although they were initially able to acquire the task (see Figures S1F and G). This deficiency was rescued by simultaneous Pik3cb knockdown (2-way ANOVA, p(Fmr1 × Pik3cb interaction)=0.04, F(1,39)=4.2). Post-hoc comparisons represent p<0.05 in all groups except for the PFC-Fmr1KD mice. Also see Figure S1E showing reduction of FMRP protein in PFC tissue punches following lentiviral shRNA knockdown, and Figure S1H showing co-expression of two viruses in the PFC.
(E) PFC-Fmr1KD mice also show greatly impaired response inhibition during extinction training, which is fully rescued by simultaneous PFC-selective Pik3cb knockdown (3-way ANOVA, p(Fmr1 × Pik3cb × session interaction)=0.05, F(1,39)=4.1; Fmr1KD mice differed significantly from all other mice on day 1 of training (all p<0.001)). As in Fmr1KO mice, Fmr1KD response rates ultimately did not differ from control mice with extended training (p=0.19, final training session of day 2). Mice with both Fmr1 and Pik3cb KD showed greater inhibitory control than mice with Fmr1 KD alone across all sessions (p<0.05).
(F) Western blot analyses of PFC tissue punches shows that knockdown of Fmr1 leads to increased mTOR phosphorylation and is reduced by Pik3cb knockdown (n(Fmr1scr/Pik3cbscr)=4, n(Fmr1KD/Pik3cbscr)=5, n(Fmr1KD/Pik3cbscr)=4, n(Fmr1KD/Pik3cbKD)=8; 2-way ANOVA, significant effect of Fmr1 and Pik3cb: p(Fmr1)=0.034, F(1, 17)=5.3; p(Pik3cb)=0.008, F(1,17)=9.2; p(interaction)=0.16, F(1,17)=2.1; Tukey’s posthoc analyses p(Fmr1KD/Pik3cbscr-Fmr1KD/Pik3cbKD)=0.01). As illustrated by the scatter blot, effects of the knockdown were variable. Additional example western blots are shown in Figure S1I.
Error bars represent SEM in A–E; and SD in F.