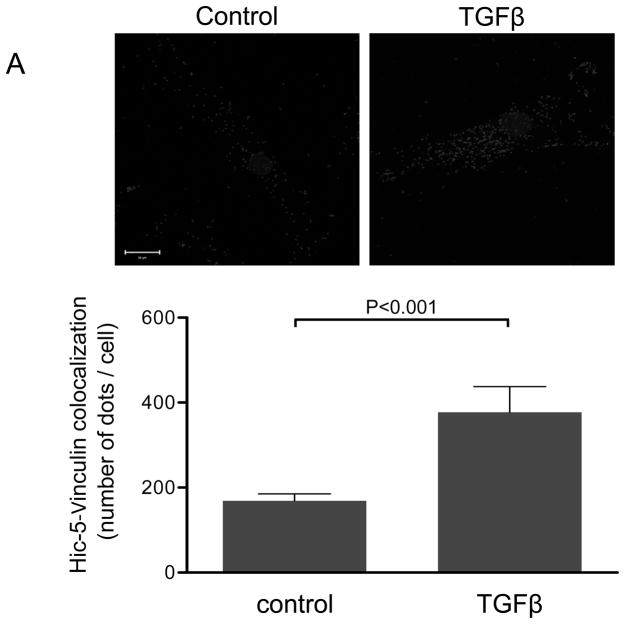
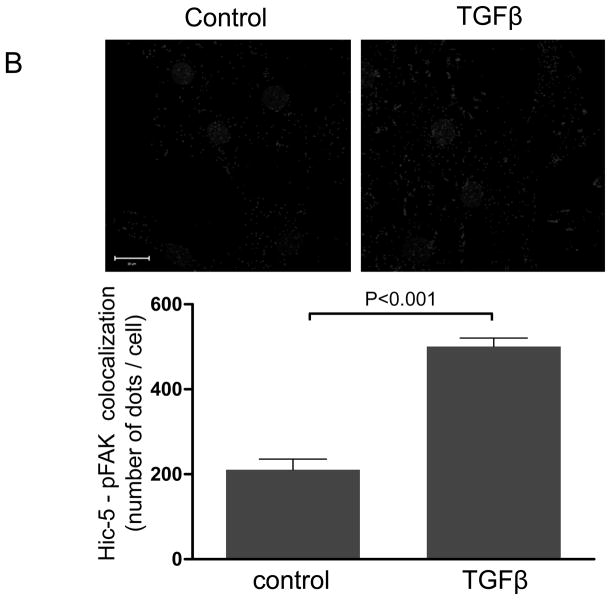
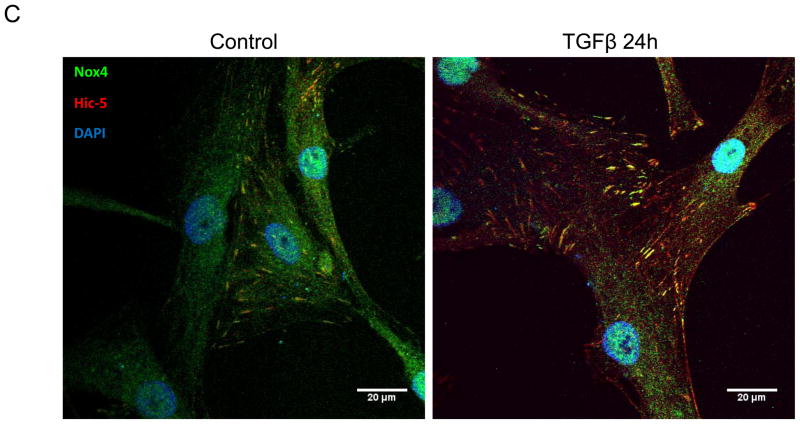
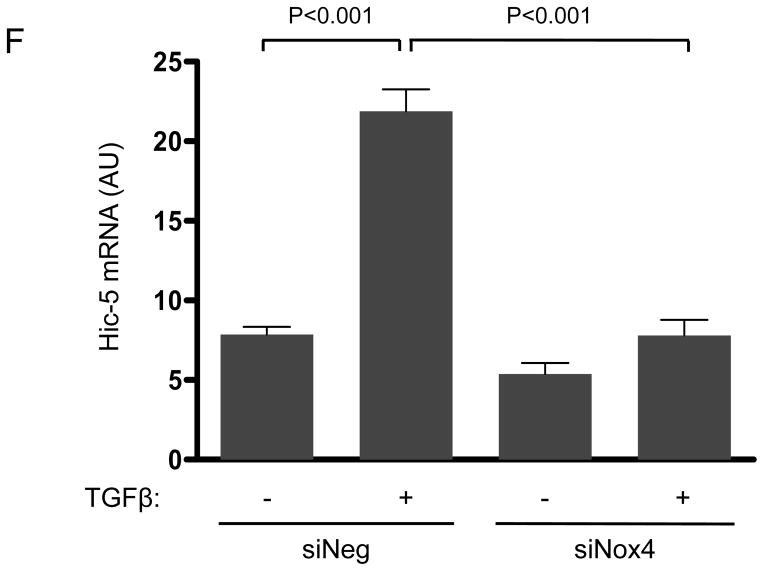
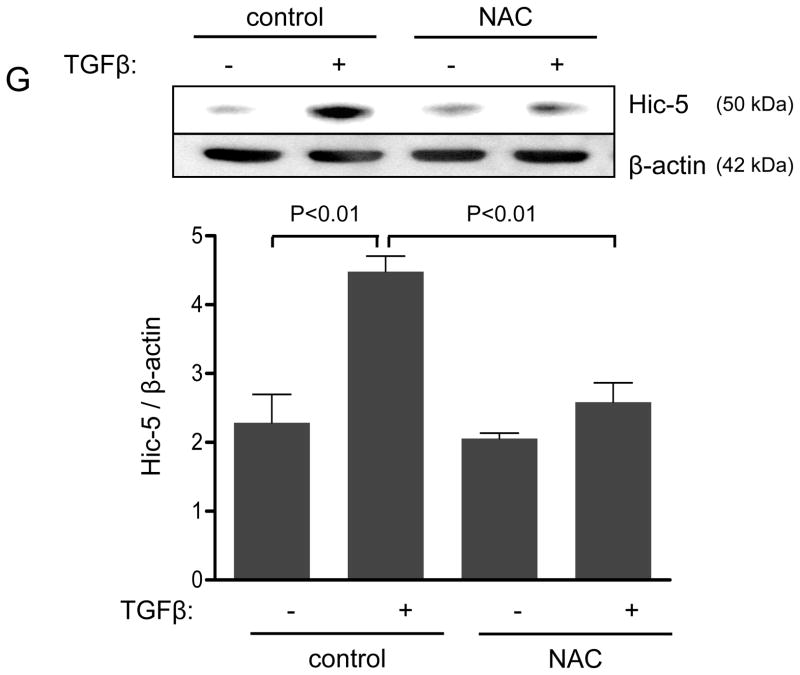
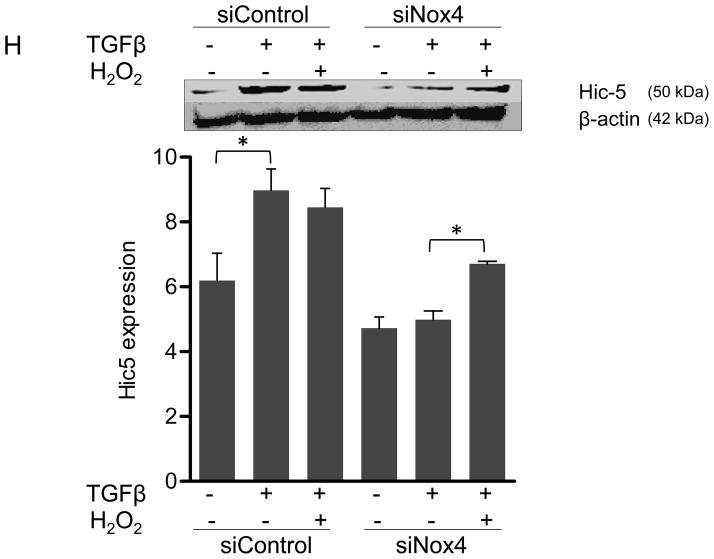
Figure 1. The focal adhesion protein Hic-5 is regulated by TGFβ via a Nox4-dependent mechanism.
Panels A-B: Hic-5 localization to FAs increases with TGFβ treatment. HASMCs were plated and maintained in serum-free medium for 2 days before treatment with TGFβ (2 ng/ml). Colocalization (red label) between Hic-5 and Vinculin (A), and Hic-5 and pFAK (B) was determined by in situ proximity ligation assay (Duolink). Panel C: Colocalization of Hic5 and Nox4 in HASMCs is increased with TGFβ treatment. HASMCs were plated and serum deprived for 2 days and then treated with TGFβ for 24h. Cells were stained for Hic-5 (red) and Nox4 (green), and nuclei were detected with DAPI (blue). Images were captured using confocal microscopy. Colocalization is evident in yellow in the right panel. Panels D-H: TGFβ increases Hic-5 expression via Nox4-produced H2O2. After 2 days of serum deprivation, HASMCs were treated with TGFβ (2 ng/mL) for indicated times and Hic-5 was detected by WB (D). HASMC were transfected with non-silencing RNA (siNeg) or siNox4 and treated with TGFβ (2 ng/mL) for 24h to analyze Hic-5 protein (E) and RNA (F) by WB and RT-qPCR respectively. (G) Cells incubated with or without N-acetyl cysteine (NAC, 10 mM) for 2 hours prior to addition of TGFβ for 24h were analyzed by western blotting. (H) HASMCs transfected with siNeg or siNox4 were treated with either TGFβ (2 ng/mL) or TGFβ (2 ng/mL) + H2O2 (50 μg/mL) and the expression of Hic-5 was analyzed by WB. All experiments were performed at least three independent times. Data is presented as average±SEM of three independent experiments.