Abstract
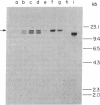
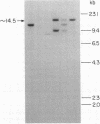
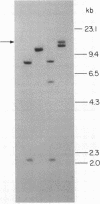
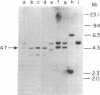
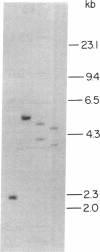
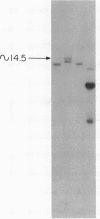
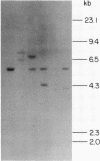
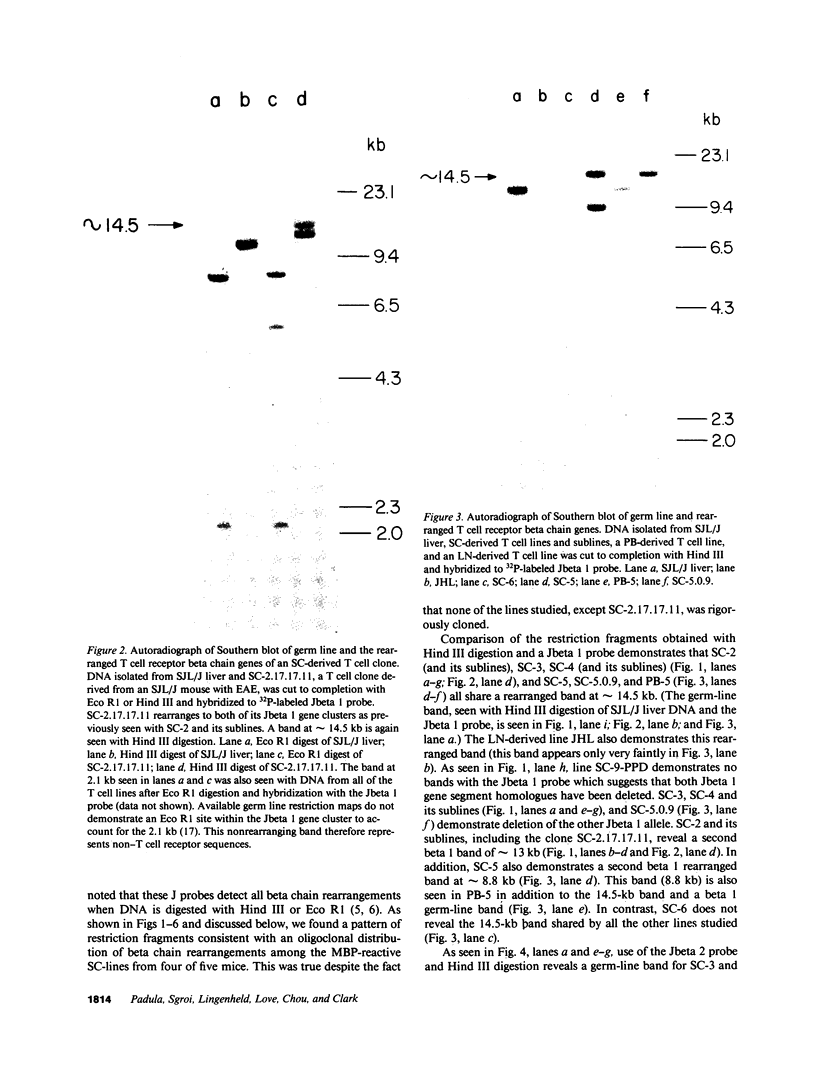
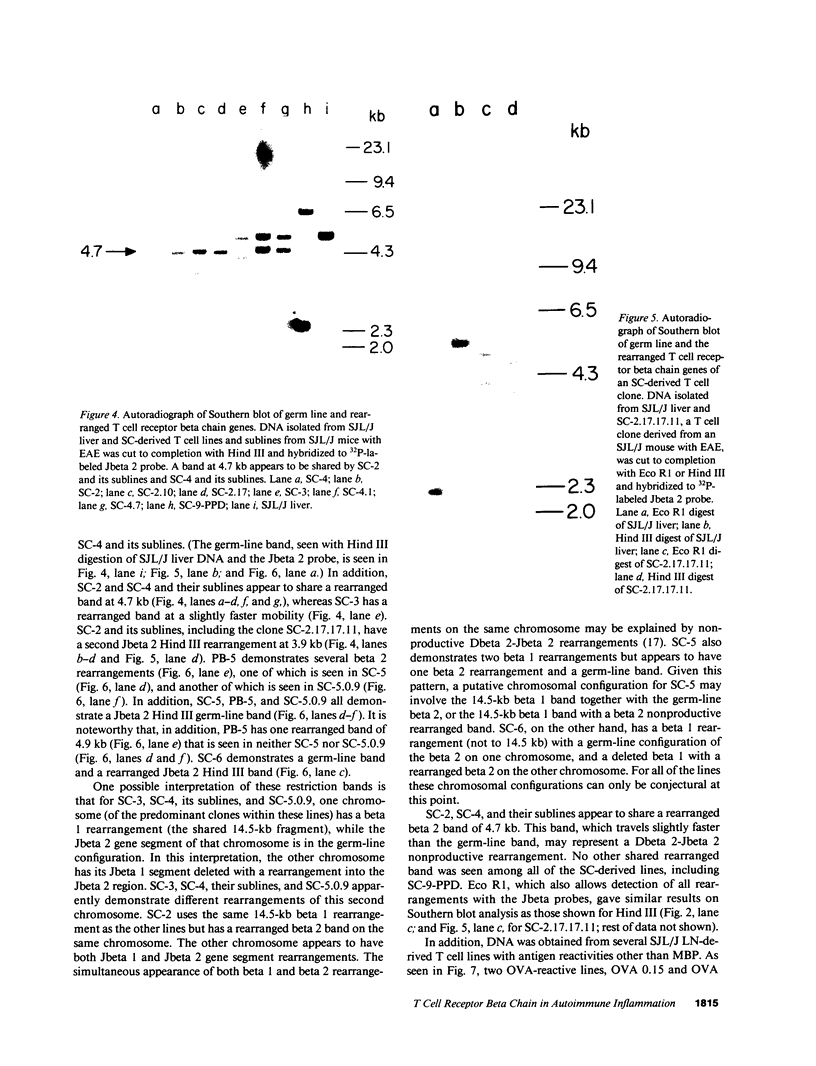
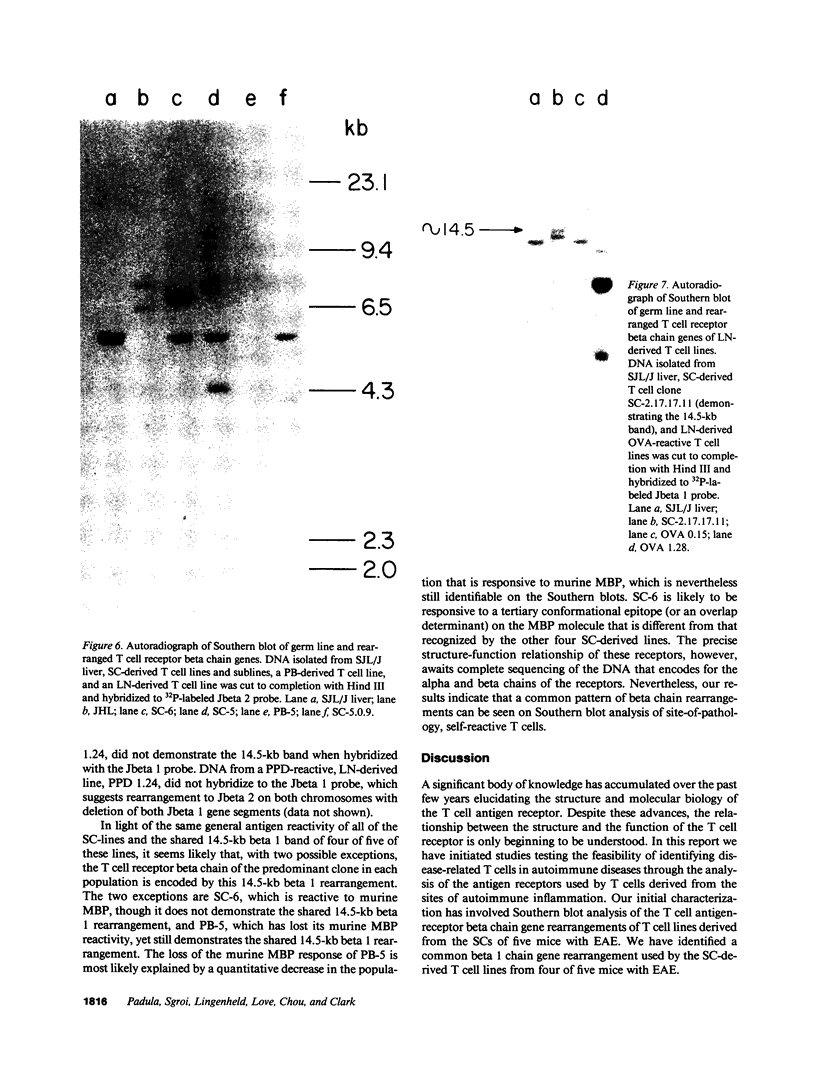
Advances in our understanding of the structure and molecular biology of the T lymphocyte antigen-receptor have now made it feasible to study human autoimmune diseases using new approaches. One such approach involves cloning of T cells from sites of autoimmune pathology followed by identification of putative disease-related T cell oligoclonality at the level of the T cell receptor gene rearrangements. We have now tested the feasibility of this approach in an animal model of autoimmunity, murine experimental allergic encephalomyelitis (EAE). Spinal cord-derived, self (murine) myelin basic protein (MBP)-reactive T cell lines and sublines were analyzed at the level of their receptor beta chain rearrangements using Southern blots. We now report that the MBP-reactive T cell lines and sublines derived from the spinal cords of four of five SJL/J mice with EAE share a 14.5-kb rearranged T cell receptor beta 1 band on Southern blots. A spinal cord-derived T cell line that was reactive to purified protein derivative of tuberculin (PPD), several lymph node-derived ovalbumin- and PPD-reactive T cell lines, as well as one MBP-reactive spinal cord-derived T cell line did not share this 14.5-kb rearranged beta 1 band. These results suggest that analysis of the antigen receptors used by T cells cloned from sites of inflammation may be a useful initial approach for identifying pathogenetically relevant T cells in the study of certain human autoimmune diseases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batchelor J. R., Compston A., McDonald W. I. The significance of the association between HLA and multiple sclerosis. Br Med Bull. 1978 Sep;34(3):279–284. doi: 10.1093/oxfordjournals.bmb.a071512. [DOI] [PubMed] [Google Scholar]
- Behlke M. A., Chou H. S., Huppi K., Loh D. Y. Murine T-cell receptor mutants with deletions of beta-chain variable region genes. Proc Natl Acad Sci U S A. 1986 Feb;83(3):767–771. doi: 10.1073/pnas.83.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B., Dore-Duffy P., Donaldson J. O., Pollard M. K., Muirhead S. P. Generation of phenotypic helper/inducer and suppressor/cytotoxic T-cell lines from cerebrospinal fluid in multiple sclerosis. Cell Immunol. 1984 Apr 1;84(2):409–414. doi: 10.1016/0008-8749(84)90113-8. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Lingenheld E. G., Donaldson J. O., Pollard M. K. Compartmentalized immune responses: antigen-specificity of cerebrospinal fluid T-cell lines maintained in the absence of antigen. Clin Immunol Immunopathol. 1985 Aug;36(2):176–186. doi: 10.1016/0090-1229(85)90119-9. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Muirhead S. P., Pollard M. K. Generation of long-term T-cell lines from synovial fluid. Clin Immunol Immunopathol. 1984 Nov;33(2):287–292. doi: 10.1016/0090-1229(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fink P. J., Matis L. A., McElligott D. L., Bookman M., Hedrick S. M. Correlations between T-cell specificity and the structure of the antigen receptor. Nature. 1986 May 15;321(6067):219–226. doi: 10.1038/321219a0. [DOI] [PubMed] [Google Scholar]
- Gusella J., Varsanyi-Breiner A., Kao F. T., Jones C., Puck T. T., Keys C., Orkin S., Housman D. Precise localization of human beta-globin gene complex on chromosome 11. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5239–5242. doi: 10.1073/pnas.76.10.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochgeschwender U., Simon H. G., Weltzien H. U., Bartels F., Becker A., Epplen J. T. Dominance of one T-cell receptor in the H-2Kb/TNP response. Nature. 1987 Mar 19;326(6110):307–309. doi: 10.1038/326307a0. [DOI] [PubMed] [Google Scholar]
- Knobler R. L., Linthicum D. S., Cohn M. Host genetic regulation of acute MHV-4 viral encephalomyelitis and acute experimental autoimmune encephalomyelitis in (BALB/cKe x SJL/J) recombinant-inbred mice. J Neuroimmunol. 1985 Apr;8(1):15–28. doi: 10.1016/S0165-5728(85)80044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M., Goverman J., Haars R., Malissen M., Kraig E., Phillips L., Delovitch T., Suciu-Foca N., Hood L. Rearrangement and transcription of the beta-chain genes of the T-cell antigen receptor in different types of murine lymphocytes. Nature. 1985 Feb 21;313(6004):647–653. doi: 10.1038/313647a0. [DOI] [PubMed] [Google Scholar]
- Norton W. T., Poduslo S. E. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973 Oct;21(4):749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Padula S. J., Pollard M. K., Lingenheld E. G., Clark R. B. Maintenance of antigen specificity by human interleukin-2-dependent T cell lines. Use of antigen-presenting cells and OKT3 antibody in the absence of antigen. J Clin Invest. 1985 Mar;75(3):788–797. doi: 10.1172/JCI111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgroi D., Cohen R. N., Lingenheld E. G., Strong M. K., Binder T., Goldschneider I., Greiner D., Grunnet M., Clark R. B. T cell lines derived from the spinal cords of mice with experimental allergic encephalomyelitis are self reactive. J Immunol. 1986 Sep 15;137(6):1850–1854. [PubMed] [Google Scholar]
- Sorger S. B., Hedrick S. M., Fink P. J., Bookman M. A., Matis L. A. Generation of diversity in T cell receptor repertoire specific for pigeon cytochrome c. J Exp Med. 1987 Feb 1;165(2):279–301. doi: 10.1084/jem.165.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978 Apr 20;298(16):869–871. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- Trotter J., Sriram S., Rassenti L., Chou C. H., Fritz R. B., Steinman L. Characterization of T cell lines and clones from SJL/J and (BALB/c x SJL/J)F1 mice specific for myelin basic protein. J Immunol. 1985 Apr;134(4):2322–2327. [PubMed] [Google Scholar]
- Zamvil S., Nelson P., Trotter J., Mitchell D., Knobler R., Fritz R., Steinman L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. 1985 Sep 26-Oct 2Nature. 317(6035):355–358. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]