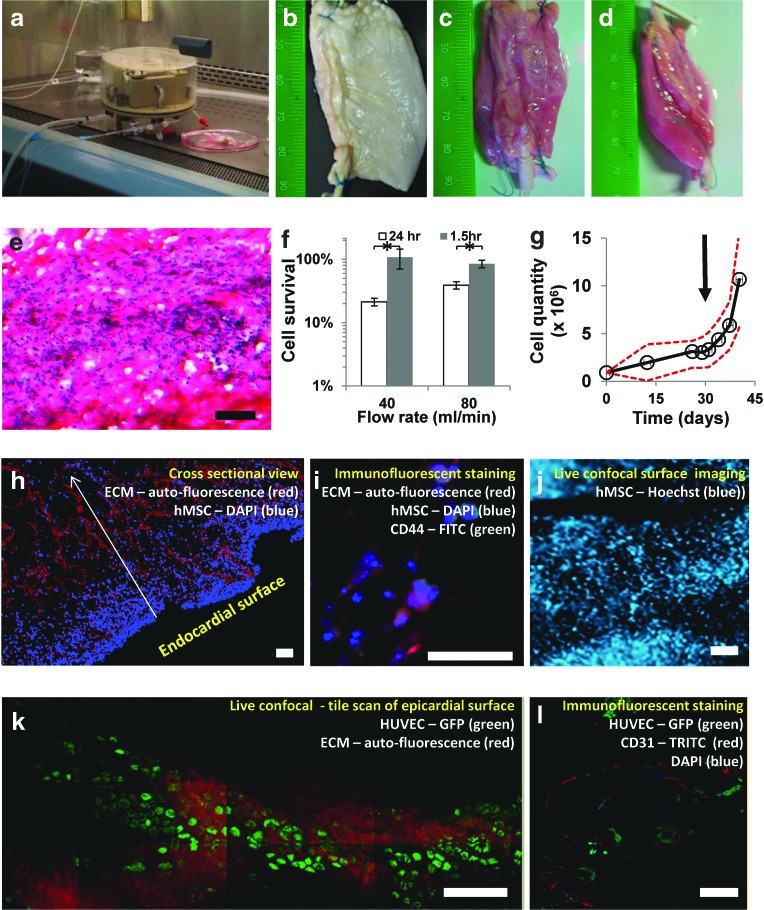
FIG. 3.
Compartmentalized dynamic recellularization using monocultures of human mesenchymal stem cells (hMSCs) and human umbilical vein endothelial cells (HUVECs). A functioning perfusion chamber can be trans-located from the CO2 incubator into a biological cabinet where sterile handling is available (a). Using this system, decellularized thick pcECM scaffolds (b) regain full thickness appearance after 48 h of perfusion, as viewed from top or side (c, d, respectively). H&E staining 7 days postseeding (e). Cell survival when cultivated under various physiological flow rates, using different seeding times (1.5 or 24 h), determined after 24 h of perfusion (f). * Denotes significantly different results p<0.05. Transferring of statically cultivated thick constructs (t=30 days, marked with an arrow) to further cultivation in the dynamic system exhibits a significant (p<0.05) increase in cell quantities (g). Dashed red line represents the 95% confidence interval of the mean. H&E staining of histological cross-sections, 7 days postdynamic cultivation of hMSCs seeded through the bulk of the pcECM by injection—fibers are shown in red and cell nuclei in blue (h). Specific antibody staining for CD44 suggests that the hMSCs are anchored to the pcECM through their HA receptors (i). Live confocal imaging (hMSCs stained with Hoechst) of the endocardial surface after 21 days of static culture reveals densely populated surfaces in accordance with the mathematical model prediction of steady state densities (j). Re-endothelialization of the vascular network within the pcECM is demonstrated using a monoculture of HUVEC-GFPs (green) forming 14 days postseeding and perfusion, a monolayer coating in a cobble stone-like formation (k). Cross-section staining of the GFP expressing cells (green) with CD31 (red) demonstrated endothelium formation within the lumen of the blood vessel (l). In all experiments, results represent three biological repetitions (n=3). Scale bars: (e), (h), (j–l), 100 μm; (i), 50 μm. Color images available online at www.liebertpub.com/tea