Abstract
With improved survival and aging, more persons living with HIV/AIDS (PLWHA) are at risk for colorectal cancer (CRC). This retrospective longitudinal study evaluated patient characteristics associated with CRC screening in our HIV cohort. Patients were followed beginning at age 50 years during a study period from January 1, 2003 to December 31, 2010 (n=265). During a median follow-up time of 1.7 years, only 30% of patients underwent CRC screening. The majority of screened patients received endoscopic screening (colonoscopy, 86%; sigmoidoscopy, 8%); among these patients, results were available for 68/75, and adenomatous polyps were found in 13%. No cases of CRC were reported. Among unscreened patients, only 23% had an external primary care provider, indicating an HIV provider was the expected source for CRC screening referral in the majority. Patients with time-varying suppressed HIV viral load were more likely to receive screening (HRadjusted=1.74; 95% CI: 1.05–2.87), independent of CD4 count. Our findings suggest HIV providers are more likely to address non-HIV-related healthcare maintenance when HIV is controlled. In addition, a significant number of neoplastic lesions are likely being missed in PLWHA who have not been screened for CRC. Provision of evidence-based preventive care in addition to HIV care is required for the aging population of PLWHA.
Due to increased longevity on combination antiretroviral therapy (cART), people living with HIV/AIDS (PLWHA) in the United States are aging, with 50% estimated to be over age 50 years by 2015.1–3 Colorectal cancer (CRC) is the third leading cause of cancer-related mortality in the United States, and risk increases with older age.4 A number of studies suggest the incidence of CRC among PLWHA may be rising, and the risk for CRC will only increase with the advancing age of this population.5–9
CRC screening has been instrumental in reducing the incidence and mortality from CRC through detection and removal of precancerous lesions.4,10 The 2008 U.S. Preventive Services Task Force guidelines recommend average risk individuals age 50–75 years receive CRC screening with either (1) high sensitivity fecal occult blood test (FOBT) annually, (2) flexible sigmoidoscopy every 5 years plus FOBT every 3 years, or (3) colonoscopy every 10 years.11 HIV primary care guidelines recommend CRC screening beginning at age 50 years.12 Although there are limited data regarding CRC among PLWHA, several studies note a higher prevalence of neoplastic lesions (precancerous adenomas or adenocarcinoma) upon asymptomatic screening compared with uninfected individuals,13–16 and some suggest HIV-infected patients with CRC are more likely to present with advanced disease.16–19 Of concern, all studies on CRC screening among PLWHA report low rates of screening.14,20–27
The studies published to date on CRC screening among PLWHA have taken a cross-sectional approach in their analyses. Many PLWHA receive primary care from an HIV provider, who must balance attention to HIV-related goals such as achieving an undetectable plasma HIV-1 RNA (VL, viral load) with primary care health maintenance. With the perspective that each HIV patient encounter beginning at age 50 represents an opportunity for referral for CRC screening, we conducted the first longitudinal study on CRC screening among PLWHA.
This retrospective analysis was set within the University of Alabama at Birmingham (UAB) 1917 Clinic Cohort (www.uab.edu/medicine/1917cliniccohort/), a prospective clinical cohort established in 1992 and described in detail elsewhere.28 Our electronic database contains detailed sociodemographic, clinical, and psychosocial data on all PLWHA receiving primary HIV care at the UAB 1917 Clinic, with care delivered by provider dyads of an infectious diseases (ID) fellow or nurse practitioner working in tandem with an ID attending physician (>6,000 patients overall with >2,000 active). The study period covered January 1, 2003 to December 31, 2010.
Patients with one or more primary HIV provider visits at age 50 years within the study period were eligible (n=319). Exclusion criteria included (1) a history of CRC, colonic polyps, colorectal surgery, or inflammatory bowel disease prior to age 50 years (n=16); (2) receipt of flexible sigmoidoscopy or air-contrast barium enema (ACBE) <5 years prior to age 50 years (n=7), or colonoscopy or unspecified CRC screening modality <10 years prior to age 50 years (n=28) as these patients were not eligible for routine CRC screening at age 50 years. Three patients with ethnicity other than white or black/African American were excluded for low numbers. The remaining 265 patients were considered eligible for CRC screening at age 50 years and were included in the analyses.
The primary outcome was time to CRC screening from age 50 years (CRC screening=colonoscopy, flexible sigmoidoscopy, ACBE, or FOBT). As this outcome is not systematically captured within our electronic database, medical record abstraction was performed by G.A.B. and L.E.A. using a standardized chart review form. A patient was defined as screened based on a procedure report or if screening was documented by a provider, whether in external records or HIV clinic or other UAB provider notes [e.g., generalist primary care provider (PCP) or gastroenterologist]. In addition, among patients who did not receive screening, documentation of referral for screening, patient refusal of referral, or HIV provider awareness of the need for screening was abstracted.
The baseline study visit was the first primary HIV provider visit at age 50 years. Independent variables included the following:
1. Sociodemographic factors: composite gender/sexual orientation [heterosexual male, men who have sex with men (MSM), or female], race/ethnicity, and insurance status at baseline.
2. Clinical factors: baseline and time-varying VL and CD4 count, time-updated cumulative arrived HIV primary care visit count, baseline smoking status, total medication count, ART status, family history of CRC in a first degree relative, and whether receiving care from a PCP external to our clinic±12 months from the baseline visit.
3. Psychosocial factors: baseline affective mental health disorder/anxiety, alcohol abuse, and substance use. These factors have been associated with decreased adherence to ART, and therefore were selected to explore if they were associated with decreased uptake of another self-care behavior, CRC screening.29–31
Family history of CRC in a first degree relative and information regarding external PCPs were obtained through medical record abstraction by G.A.B. and L.E.A. All other independent variables were obtained by query (MS SQL) of the UAB 1917 Clinic Cohort electronic database. This study was approved by the UAB Institutional Review Board.
Initial descriptive analyses included comparison of those screened versus not screened. Continuous variables were reported as median with quartiles (first, Q1 and third, Q3). Categorical variables were reported as frequency with percentages. Kaplan–Meier curves were constructed to evaluate time to CRC screening. Patients were censored at first CRC screening, death, dropping out of care (no HIV provider visit for 18 months with censor date being that of the last visit), or at the end of the study period. Cox proportional hazard models reporting hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were used to test associations among independent variables and time to screening. Statistical significance was set at 0.05 (two-tailed). Clinically relevant variables selected a priori for the multivariable model included gender/sexual orientation, race/ethnicity, baseline insurance status, family history of CRC in first degree relative, external PCP±12 months from baseline visit, and time-varying VL and CD4 count. Analyses were conducted using SAS statistical software (version 9.3).
Among the 265 study participants, 79% were male and 40% were black/African American (Table 1). Most were insured (40% private and 41% public insurance). Approximately 25% of patients had documented evidence of an external PCP±12 months from their baseline age 50 years visit.
Table 1.
Factors Associated with Colorectal Cancer Screening Among Patients Living with HIV and AIDS Receiving Care at UAB 1917 Clinic from Age 50 Years Between January 1, 2003 and December 31, 2010
| Overall study population (N=265) | Received CRC screening (N=80) | Did not receive CRC screening (N=185) | Univariate analysisb | Multivariable analysisc | |||
|---|---|---|---|---|---|---|---|
| Characteristic | n (%)a | n (%)a | n (%)a | Unadjusted HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value |
| Gender/sexual orientation | |||||||
| Women | 56 (21.2) | 13 (16.2) | 43 (23.4) | REF | — | REF | — |
| Heterosexual men | 51 (19.3) | 14 (17.5) | 37 (20.1) | 1.45 (0.68–3.07) | 0.34 | 1.76 (0.81–3.82) | 0.16 |
| MSM | 157 (59.5) | 53 (66.3) | 104 (56.5) | 1.68 (0.92–3.09) | 0.09 | 2.03 (1.04–3.99) | 0.04 |
| Race/ethnicity | |||||||
| White | 158 (59.6) | 47 (58.7) | 111 (60.0) | REF | — | REF | — |
| Black/African American | 107 (40.4) | 33 (41.3) | 74 (40.0) | 0.83 (0.53–1.30) | 0.43 | 1.13 (0.68–1.88) | 0.65 |
| Baseline insurance status | |||||||
| None | 52 (19.6) | 11 (13.8) | 41 (22.2) | REF | — | REF | — |
| Private | 105 (39.6) | 37 (46.2) | 68 (36.7) | 1.46 (0.74–2.86) | 0.27 | 1.52 (0.75–3.07) | 0.24 |
| Public | 108 (40.8) | 32 (40.0) | 76 (41.1) | 1.24 (0.62–2.46) | 0.54 | 1.52 (0.74–3.11) | 0.25 |
| Family history of CRCd | 16 (6.0) | 6 (7.5) | 10 (5.4) | 1.35 (0.59–3.11) | 0.48 | 1.49 (0.64–3.47) | 0.35 |
| External PCPe | 65 (24.5) | 23 (28.8) | 42 (22.7) | 1.33 (0.82–2.17) | 0.24 | 1.27 (0.77–2.09) | 0.35 |
| Tobacco usef | 117 (44.2) | 29 (36.3) | 88 (47.6) | 0.72 (0.45–1.13) | 0.15 | — | — |
| Alcohol abusef | 28 (10.6) | 9 (11.3) | 19 (10.3) | 1.03 (0.52–2.06) | 0.93 | — | — |
| Substance usef | 39 (14.7) | 6 (7.5) | 33 (17.8) | 0.50 (0.22–1.16) | 0.11 | — | — |
| Affective mental health disorder/anxietyf | 132 (49.8) | 39 (48.8) | 93 (50.3) | 1.01 (0.65–1.57) | 0.96 | — | — |
| Medication countf | |||||||
| ≥11 | 49 (18.5) | 11 (13.8) | 38 (20.5) | REF | — | ||
| 6–10 | 98 (37.0) | 33 (41.2) | 65 (35.2) | 1.54 (0.78–3.04) | 0.22 | — | — |
| ≤5 | 118 (44.5) | 36 (45.0) | 82 (44.3) | 1.18 (0.60–2.33) | 0.63 | ||
| On ARTf | 210 (79.3) | 61 (76.3) | 149 (80.6) | 0.90 (0.54–1.51) | 0.69 | — | — |
| Plasma HIV-1 RNA <50 copies/mlf | 144 (60.5) | 43 (58.1) | 101 (61.6) | 1.17 (0.70–1.78) | 0.64 | — | — |
| Time-varying plasma HIV-1 RNA <50 copies/ml | — | — | — | 1.61 (0.99–2.62) | 0.06 | 1.75 (1.07–2.88) | 0.03 |
| CD4 count <200 cells/μlf | 35 (14.5) | 11 (14.7) | 24 (14.5) | 0.85 (0.45–1.62) | 0.63 | — | — |
| Time-varying CD4 count <200 cells/μl | — | — | — | 1.00 (1.00–1.00) | 0.89 | — | — |
| Time-updated cumulative arrived HIV primary care visits | — | — | — | 1.03 (0.94–1.14) | 0.51 | — | — |
Column percents.
Univariate Cox proportional hazards model.
Multivariable Cox proportional hazards model with clinically relevant variables selected a priori.
In first degree relative (at baseline).
Within±12 months of baseline visit.
At baseline age 50 years visit.
Bold typeface indicates statistical significance at 0.05 level.
ART, antiretroviral therapy; CI, confidence interval; CRC, colorectal cancer; HIV, human immunodeficiency virus; HR, hazard ratio; MSM, men who have sex with men; PCP, primary care provider; PLWHA, persons living with HIV and AIDS.
Missing data: sexual orientation=1; baseline CD4 count=24; baseline plasma HIV-1 RNA=27.
The median follow-up time was 1.7 years. Only 80 patients (30%) received CRC screening during the study period, with a median time to screening from age 50 years of 1.5 years (Q1–Q3: 0.6–2.4 years). There was no significant difference in time to screening based on era of entry into the study (2003–2005, 2006–2008, or 2009–2010; p=0.52). Mortality did not differ significantly between screened and unscreened patients (4% versus 7%; p=0.56). Of the 80 patients screened, 60 (75%) had CRC testing ordered for routine screening, 13 (16%) for diagnostic purposes, and 7 (9%) for undocumented reasons. Of those screened 46 (58%) were referred by a primary HIV provider and 22 (28%) by an external provider; the referral source was unavailable for the remainder. Screening was performed by colonoscopy in 69 (86%) and flexible sigmoidoscopy in 6 (8%); results were available for 68 patients, of whom 9 (13%) had neoplastic lesions, all tubular adenomas.
Of the 185 patients who did not receive CRC screening, 33 (18%) had been referred for screening, 6 (3%) refused screening, in 35 cases (19%) an HIV provider documented the need for screening but did not refer, and in 111 cases (60%) there was neither referral nor documentation regarding the need for CRC screening. Only 23% of unscreened patients had documentation of an outside PCP.
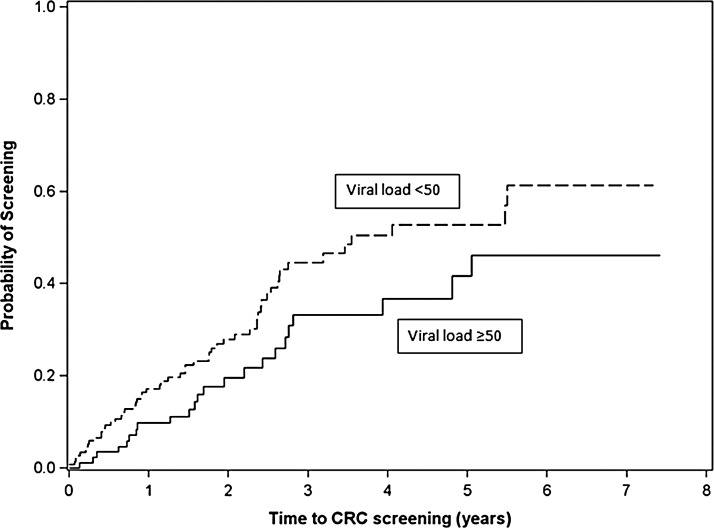
In multivariable analysis (Table 1), men who have sex with men (MSM) were more likely to be screened (HRadjusted=2.03; 95% CI: 1.04–3.99). Time-varying suppressed VL (<50 copies/ml) was significantly associated with more prompt CRC screening (HRadjusted=1.75; 95% CI: 1.07–2.88) (Table 1 and Fig. 1). The association between time-varying suppressed VL and CRC screening remained significant (HRadjusted=1.74; 95% CI: 1.05–2.87) when time-varying CD4 count (<200 vs. ≥200 cells/μl) was included (model not shown), whereas time-varying CD4 count <200 cells/μl showed no significant association with CRC screening (HRadjusted=0.92; 95% CI: 0.46–1.85). In addition, these findings were robust in a sensitivity analysis in which patients referred but not screened were counted as screened and censored at time of referral (model not shown).
FIG. 1.
Time to colorectal cancer screening from age 50 years for UAB 1917 HIV Clinic patients (N=265) with suppressed versus unsuppressed time-varying plasma HIV-1 RNA (VL, viral load). p=0.06.
In this first longitudinal study on CRC screening among PLWHA, we found screening per USPSTF recommendations is underutilized in our cohort, with 70% of patients not screened during a median of 1.7 years of follow-up from age 50 years. Among unscreened patients, only 23% had an external PCP, indicating an HIV provider was the expected source for CRC screening referral in the majority. Most unscreened patients had neither evidence of referral nor documentation of the need for screening in their records, suggesting CRC screening is often not explicitly addressed by HIV providers. We found that patients with viral suppression (<50 copies/ml) were significantly more likely to receive CRC screening over time, perhaps related to an increased ability or willingness among providers to address non-HIV-related health maintenance when HIV is controlled.
Our study is unique in its longitudinal design and use of survival methods to evaluate the relationship between patient characteristics and CRC screening starting from the time guidelines recommend routine screening (i.e., age 50 years). We adopted this approach for two compelling reasons. First, each encounter with an HIV provider represents an opportunity for CRC screening referral. A cross-sectional design fails to capture the differing opportunities a patient in care for 6 months versus 5 years would have for referral and receipt of CRC screening. Second, with each encounter HIV providers theoretically are balancing HIV-related goals such as achieving an undetectable VL with primary care. This led us to use viral suppression as a time-varying rather than baseline variable. When baseline VL at age 50 years was substituted for time-varying VL in our multivariable model, no association with CRC screening was observed (HRadjusted=1.32; 95% CI: 0.82–2.12), suggesting the most recent VL available to a provider plays a more important role in CRC screening referral than the first VL value when someone turns 50 years.
The low rate of screening we observed is consistent with prior studies, in which the proportion of PLWHA receiving CRC screening ranges from 25% to 64%.14,20–27 While low rates of screening also occur within the general population, PLWHA may have disproportionately lower rates. Several studies directly comparing screening between PLWHA and uninfected patients note a significantly lower proportion of PLWHA screened (50% vs. 71% in Iqbal et al., 56 vs. 78% in Reinhold et al., and 54 vs. 65% in Momplaisir et al.).14,22,23
Results from a recent study among Medicaid patients found age- and gender-matched HIV-infected patients were more likely to receive CRC screening than uninfected controls, however, when adjustment was made for comorbidities and years in the dataset, HIV-infected patients were 20% less likely to receive screening.26 Despite improved survival, the population of PLWHA in the United States still experiences excess mortality compared to the general population,32 and it is largely unknown whether CRC screening has comparable risks and benefits among PLWHA. However, we note that HIV primary care guidelines have recommended that PLWHA should be managed according to standard practices in the United States for their age and sex since they were first published in 2004.33 The guidelines do not address the impact of viremia on screening decisions.
Ours is the first study on CRC screening among PLWHA to evaluate sexual orientation and we found that MSM were more likely to receive CRC screening after adjusting for race/ethnicity, family history of CRC, having an external PCP, time-varying suppressed VL, and insurance status. One possible explanation is that MSM may report more lower gastrointestinal tract symptoms leading to a referral to a gastroenterology clinic or for lower endoscopy. In addition, MSM may be more likely to be referred to a colorectal surgeon for anal pathology such as human papillomavirus-related perianal warts or dysplasia detected on routine anal Papanicolaou smear, and subsequently receive colonoscopy.
Our study has limitations. Results from a single academic center in the southeastern United States may not be generalizable to other HIV clinical settings or geographic areas. The median follow-up time was relatively short at 1.7 years. Our modest sample size may have limited our ability to detect statistically significant associations. CRC screening is not systematically captured in our electronic database and was extracted by medical records review. For CRC screening done outside UAB, we were dependent on the presence of external records, which are often incomplete, or HIV provider documentation of screening, which may result in underreporting. However, we note the proportion of our patients screened is within the range observed in other studies.
Utilizing a unique longitudinal study design evaluating CRC screening from the time patients became eligible, we corroborated the low rates of screening observed in other studies, and generated novel findings regarding the increased likelihood of screening among MSM and patients with time-varying viral suppression. Our findings suggest that HIV providers may defer primary care health maintenance when HIV is uncontrolled, irrespective of CD4 count. Suboptimal CRC screening is not unique among recommended preventive health and comorbidity management for PLWHA. Suboptimal achievement of other primary care health maintenance has been observed among PLWHA,23,25,34 as well as suboptimal management of non-HIV-related comorbidities.35–38 Given the aging of the HIV-infected population with the accompanying increased risk for non-HIV-related chronic diseases and that many PLWHA depend upon their HIV provider for primary care, increased efforts are needed at every level—healthcare policy, research, HIV provider education and training, and patient-centered interventions—to help HIV providers adapt to their evolving role as caretakers of an aging population and improve primary care among PLWHA.
Acknowledgments
Special thanks to Suneetha Thogaripolly for data retrieval and to Kenneth Saag, MD, MSc, Monika M. Safford, MD, Ryan Outman, MS, and the UAB Center for Outcomes and Effectiveness Research and Education (COERE). We thank the UAB 1917 Clinic Cohort staff and management for their assistance with this project (www.uab1917cliniccohort.org).
Sources of support: G.A.B. was supported in part by the Agency for Healthcare Research and Quality (Grant 5T32HS013852). This work was supported by the UAB Center for AIDS Research (P30-AI27767), CNICS (1R24 AI067039-1), the Mary Fisher CARE Fund, and a Bristol Myers-Squibb Virology Research Fellows training grant to G.A.B.
Data were presented in part at the Society of General Internal Medicine 34th Annual Meeting, Orlando, FL, May 9–12, 2012.
Author Disclosure Statement
G.A.B. received research support from the Bristol-Myers Squibb Virology Fellows Research Training Program and is a consultant for Definicare, LLC and Amgen. J.H.W. has received research support from Bristol-Myers Squibb, Pfizer, Tibotec Therapeutics, and Definicare, LLC, and has consulted for Bristol-Myers Squibb and Gilead Sciences. M.S.S. has received grant support and/or has consulted for Boehringer Ingelheim Pharmaceuticals, Inc., Bristol-Myers Squibb, Gilead Sciences, Inc., GlaxoSmithKline, Merck & Co, Inc., Pfizer Inc., Vertex Pharmaceuticals, Inc., and ViiV Healthcare. He has an equity ownership in Definicare, LLC. A.O.W. has consulted for Definicare, LLC. M.J.M. has received consulting fees (advisory board) from Merck Foundation, Bristol-Myers Squibb, and Gilead Sciences, and grant support to UAB from Tibotec Therapeutics, Pfizer, Inc., Bristol-Myers Squibb, and Definicare, LLC.
References
- 1.Palella FJ, Jr, Baker RK, Moorman AC, et al. : Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV Outpatient Study. J Acquir Immune Defic Syndr 2006;43:27–34 [DOI] [PubMed] [Google Scholar]
- 2.The Antiretroviral Therapy Cohort Collaboration: Life expectancy of individuals on combination antiretroviral therapy in high income countries: A collaborative analysis of 14 cohort studies. Lancet 2008;372:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Effros RB, Fletcher CV, Gebo K, et al. : Workshop on HIV infection and aging: What is known and further research directions. Clin Infect Dis 2008;47:542–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, and Jemal A: Cancer Statistics, 2013. CA Cancer J Clin 2013;63:11–30 [DOI] [PubMed] [Google Scholar]
- 5.Shiels MS, Pfeiffer RM, Gail MH, et al. : Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011;103:753–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polesel J, Franceschi S, Suligol B, et al. : Cancer incidence in people with AIDS in Italy. Int J Cancer 2010;127:1437–1445 [DOI] [PubMed] [Google Scholar]
- 7.Patel P, Hanson DL, Sullivan PS, et al. : Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 2008;148:728–736 [DOI] [PubMed] [Google Scholar]
- 8.Silverberg MJ, Chao C, Leyden WA, et al. : HIV infection and the risk of cancers with and without a known infectious cause. AIDS 2009;23:2337–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedimo R, Chen RY, Accortt NA, et al. : Trends in AIDS-defining and non-AIDS-defining malignancies among HIV-infected patients: 1998–2002. Clin Infect Dis 2004;39:1380–1384 [DOI] [PubMed] [Google Scholar]
- 10.Edwards BK, Ward E, Kohler BA, et al. : Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Preventive Services Task Force: Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2008;149:627–637 [DOI] [PubMed] [Google Scholar]
- 12.Aberg JA, Gallant JE, Ghanem KG, et al. . and the Infectious Diseases Society of America: Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014;58:1–10 [DOI] [PubMed] [Google Scholar]
- 13.Gutkin E, Hussain SA, Mehta P, et al. : Prevalence of adenomas found on colonoscopy in patients with HIV. Gastroenterol Res 2012;5:52–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iqbal S, Browne-McDonald V, and Cerulli MA: Recent trends for colorectal cancer screening in HIV-infected patients. Dig Dis Sci 2010;55:761–766 [DOI] [PubMed] [Google Scholar]
- 15.Bini EJ, Park J, and Francois F: Use of flexible sigmoidoscopy to screen for colorectal cancer in HIV-infected patients 50 years of age and older. Arch Intern Med 2006;166:1626–1631 [DOI] [PubMed] [Google Scholar]
- 16.Bini EJ, Green B, and Poles MA: Screening colonoscopy for the detection of neoplastic lesions in asymptomatic HIV-infected subjects. Gut 2009;58:1129–1134 [DOI] [PubMed] [Google Scholar]
- 17.Berretta M, Cappellani A, Di Benedetto F, et al. : Clinical presentation and outcome of colorectal cancer in HIV-positive patients: A clinical case-control study. Onkologie 2009;32:319–324 [DOI] [PubMed] [Google Scholar]
- 18.Wasserberg N, Nunoo-Mensah JW, Gonzalez-Ruiz C, et al. : Colorectal cancer in HIV-infected patients: A case-control study. Int J Colorectal Dis 2007;22:1217–1221 [DOI] [PubMed] [Google Scholar]
- 19.Chapman C, Aboulafia DM, Dezube BJ, and Pantanowitz L: Human immunodeficiency virus-associated adenocarcinoma of the colon: Clinicopathological findings and outcome. Clin Colorectal Cancer 2009;8:215–219 [DOI] [PubMed] [Google Scholar]
- 20.Nayudu SK. and Balar B: Colorectal cancer screening in human immunodeficiency virus population: Are they at average risk? World J Gastrointest Oncol 2012;4:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Momplaisir F, Long JA, Badolato G, and Brady KA: The role of primary care physicians in improving colorectal cancer screening in patients with HIV. J Gen Intern Med 2012;27:940–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinhold JP, Moon M, Tenner CT, et al. : Colorectal cancer screening in HIV-infected patients 50 years of age and older: Missed opportunities for prevention. Am J Gastroenterol 2005;100:1805–1812 [DOI] [PubMed] [Google Scholar]
- 23.Momplaisir F, Mounzer K, and Long JA: Preventive cancer screening practices in HIV-positive patients. AIDS Care 2014;26:87–94 [DOI] [PubMed] [Google Scholar]
- 24.Campbell J. and Young B: Use of screening colonoscopy in ambulatory HIV-infected patients. J Int Assoc Physicians AIDS Care 2008;7:286–288 [DOI] [PubMed] [Google Scholar]
- 25.Sheth AN, Moore RD, and Gebo KA: Provision of general and HIV-specific health maintenance in middle aged and older patients in an urban HIV clinic. AIDS Patient Care STDS 2006;20:318–327 [DOI] [PubMed] [Google Scholar]
- 26.Momplaisir F, Keller S, Lo Re V III, et al. : Colorectal cancer incidence and screening in U.S. Medicaid patients with and without HIV infection. AIDS Care 2014;26:716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guest JL, Rentsch CT, and Rimland D: Comparison of colorectal cancer screening and diagnoses in HIV-positive and HIV-negative veterans. AIDS Care 2014;26:1490–1493 [DOI] [PubMed] [Google Scholar]
- 28.Willig JH, Aban I, Nevin CR, et al. : Darunavir outcomes study: Comparative effectiveness of virologic suppression, regimen durability, and discontinuation reasons for three-class experienced patients at 48 weeks. AIDS Res Hum Retroviruses 2010;26:1279–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langebeek N, Gisolf EH, Reiss P, et al. : Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: A meta-analysis. BMC Med 2014;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azar MM, Springer SA, Meyer JP, and Altice FL: A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend 2010;112:178–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uthman OA, Magidson JF, Safren SA, and Nachega JB: Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: A systematic review and meta-analysis. Curr HIV/AIDS Rep 2014;11:291–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antiretroviral Therapy Cohort Collaboration, Zwahlen M, Harris R, May M, et al. : Mortality of HIV-infected patients starting potent antiretroviral therapy: Comparison with the general population in nine industrialized countries. Int J Epidemiol 2009;38:1624–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aberg JA, Gallant JE, Anderson J, et al. : Primary care guidelines for the management of persons infected with human immunodeficiency virus: Recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2004;39:609–629 [DOI] [PubMed] [Google Scholar]
- 34.Burkholder GA, Tamhane AR, Salinas JL, et al. : Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clin Infect Dis 2012;55;1550–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinsch N, Neuhaus K, Esser S, et al. : Are HIV patients undertreated? Cardiovascular risk factors in HIV: Results of the HIV-HEART Study. Eur J Cardiovasc Prev Rehabil 2012;19:267–274 [DOI] [PubMed] [Google Scholar]
- 36.Freilberg MS, Leaf DA, Goulet JL, et al. : The association between the receipt of lipid lowering therapy and HIV status among veterans who met NCEP/ATP III criteria for the receipt of lipid-lowering medication. J Gen Intern Med 2009;24:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willig JH, Jackson DA, Westfall AO, et al. : Clinical inertia in the management of low-density lipoprotein abnormalities in an HIV clinic. Clin Infect Dis 2008;46:1315–1318 [DOI] [PubMed] [Google Scholar]
- 38.Adeyemi O, Vibhakar S, and Max B: Are we meeting the American Diabetes Association goals for HIV-infected patients with diabetes mellitus? Clin Infect Dis 2009;49:799–802 [DOI] [PubMed] [Google Scholar]