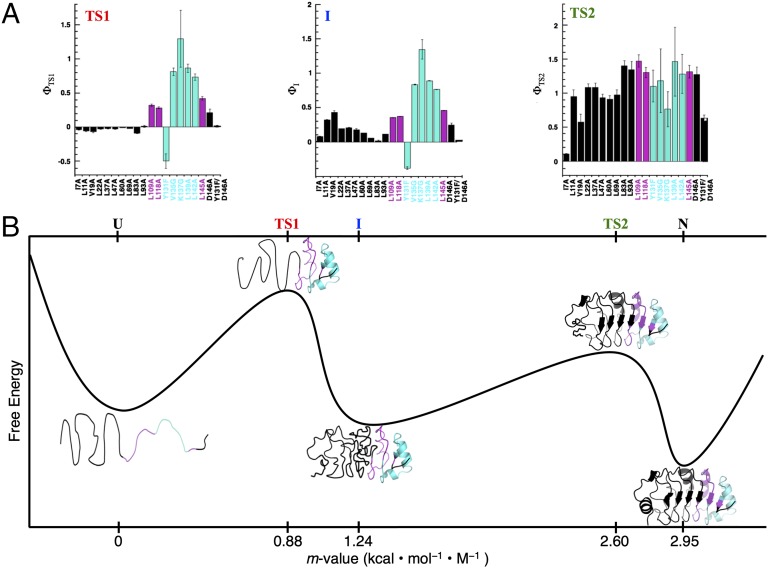
Fig. 5.
Φ-values and the folding pathway of PP32. (A) Folding Φ-values for the first (rate-limiting) transition state (TS1), intermediate (I), and second transition state (TS2). Residues that are structured (Φ > 0.5), partially structured (0.25 < Φ < 0.5), and unstructured (Φ < 0.25) in TS1 are in cyan, magenta, and black, respectively. (B) A reaction coordinate for folding of PP32. The structural models of TS1, I, and TS2 are derived from the Φ-values in A. The location of each successive state (from D to N) is based on kinetic m-values (the sum of the m-values of preceding steps, as determined from the sequential three-state fit of the WT PP32 kinetic data; Table 1).