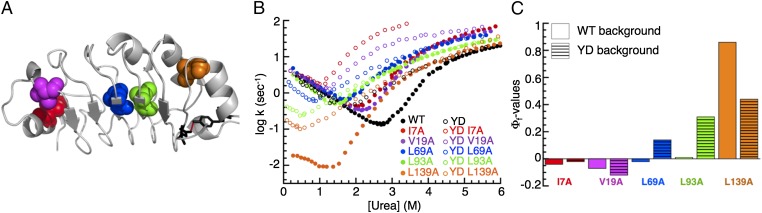
Fig. 8.
Effects of destabilizing the C terminus to the folding pathway of PP32. (A) Ribbon representation of PP32. Residues Y131 and D146, which we have substituted to destabilize the C terminus, are shown as black sticks. Residues substituted in Φ-value analysis in the Y131F/D146L (YD) background are shown in sphere representation. (B) Urea dependence of fluorescence-monitored rate constants for the major refolding and unfolding phases of variants in WT and YD backgrounds. (C) Φ-values for TS1 in the WT and YD backgrounds. The transition state for the YD construct is less polarized than for WT PP32.