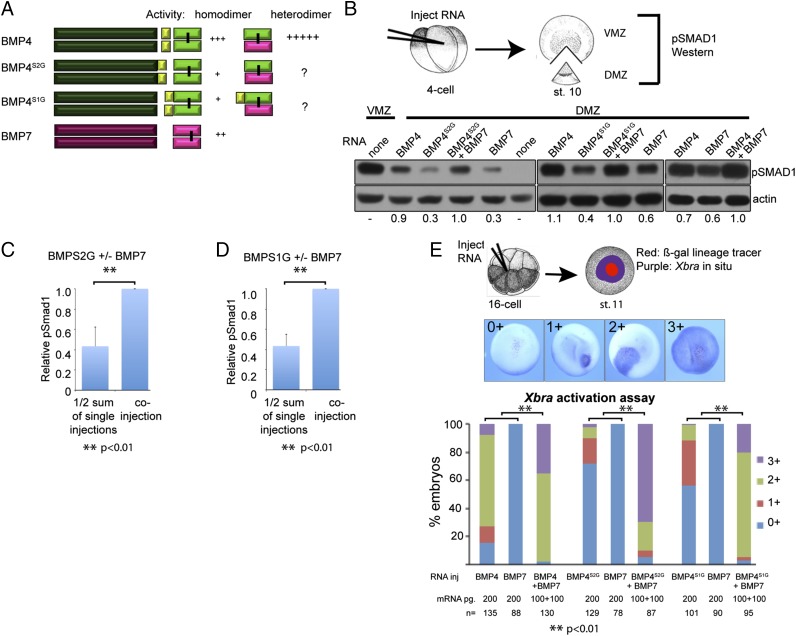
Fig. 2.
Sequential cleavage of proBMP4 is required for maximal activity of homodimeric but not heterodimeric ligands. (A) Schematic illustration of cleavage patterns and relative bioactivity of ligands generated from wild-type and cleavage mutant BMP precursor proteins. (B) RNA encoding BMP7 or wild type or cleavage mutant forms of BMP4 were injected alone (200 pg) or in combination (100 pg of each RNA) near the dorsal midline of four-cell embryos. DMZ and VMZ explants were isolated at stage 10 and pSMAD1 levels were analyzed by immunoblot. Blots were reprobed with actin as a loading control, with the exception of the right panel, in which case duplicate samples were rerun and probed with actin. Blots shown in each of the three panels were run on different days, using samples from independent experiments, and exposed for different time periods. Thus, results can only be compared within each panel. For each panel, the relative level of pSMAD1 induced by injection of BMP4 or BMP7 alone, normalized to actin, and reported as a fraction of that induced by the two coinjected RNAs is indicated below each lane. (C and D) Quantitation of relative pSMAD1 levels in three independent experiments (mean ± SD). Normalized pSMAD1 levels in embryos injected with BMP7 or BMP4S2G alone (C), or with BMP7 or BMP4S1G alone (D) were added together, divided by two, and reported as a fraction of pSMAD1 levels induced by the two coinjected RNAs. (E) BMP RNAs were injected along with β-galactosidase RNA into one animal pole blastomere of 8- to 16-cell embryos. Embryos were stained for β-galactosidase activity (red) at stage 11 and then assayed for expression of Xbra by in situ hybridization (purple) as illustrated. The spread of Xbra signal outside of cells that express the precursor proteins (marked by red) was scored as 0+ to 3+ as illustrated. A total of 30–50 embryos were injected with each RNA per experiment and results are pooled from a minimum of three experiments.