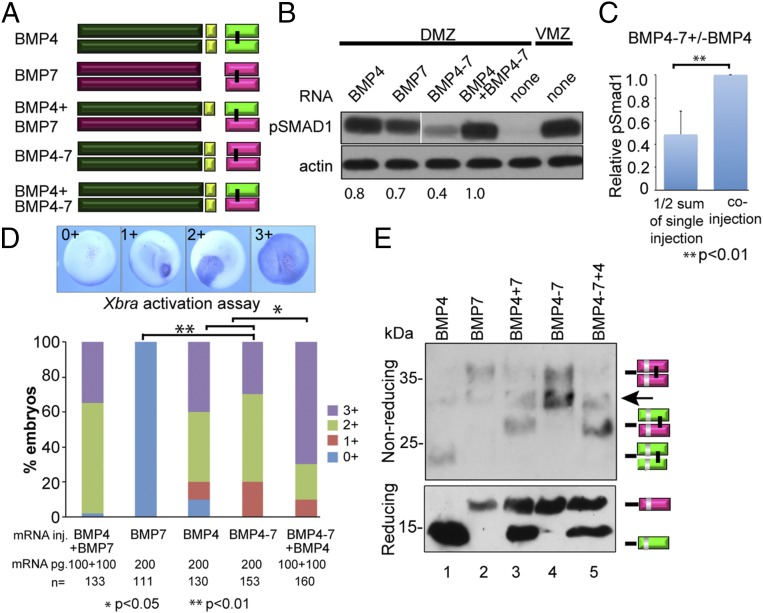
Fig. 4.
The prodomain of BMP4 is necessary and sufficient to generate stable heterodimers with enhanced activity. (A) Schematic illustration of ligands generated from native and chimeric BMP precursor proteins. (B) BMP RNAs were injected individually (200 pg) or together (100 pg of each RNA) near the dorsal midline of four-cell embryos. DMZ and VMZ explants were isolated at stage 10 and pSMAD1 levels were analyzed by immunoblot. Actin levels were analyzed in duplicate samples as a loading control. The relative level of pSMAD1 induced by injection of BMP4-7 or BMP4 alone, normalized to actin and reported as a fraction of that induced by the two co-injected RNAs, is indicated below each lane. All lanes are from the same pSmad1 immunoblot, aligned following removal of an intervening lane (following the 2nd lane, marked by white bar) using Photoshop. The pSmad1 data shown in Figs. 2B (Lower Right), 3B, and 4B is all from a single injection experiment, run on the same gel and thus the BMP4 and BMP7 control lanes are the same in all three blots. Actin loading controls for these lanes are from duplicate samples that were rerun on different gels. (C) Normalized pSMAD1 levels in embryos injected with BMP4–7 or BMP4 alone were added together, divided by two, and reported as a fraction of pSMAD1 levels induced by the two coinjected RNAs. Data are from three independent experiments (mean ± SD). (D) BMP RNAs were injected along with β-galactosidase RNA into one animal pole blastomere of 8- to 16-cell embryos. Embryos were stained for β-galactosidase activity at stage 11 and then assayed for expression of Xbra by in situ hybridization. The spread of Xbra signal outside of cells that express the precursor proteins was scored as 0+ to 3+ as illustrated. A total of 30–50 embryos were injected with each RNA per experiment and results are pooled from a minimum of three experiments. (E) Protein present in blastocele fluid from the same number of embryos in each group was deglycosylated, resolved by SDS/PAGE under reducing or nonreducing conditions as indicated, and immunoblots were probed with antibodies specific for the myc epitope in the ligand domain. Bands corresponding to each ligand dimer or monomer are indicated to the right of each gel. The arrow marks a nonspecific band due to cross-reaction of the myc antibody with PNGase.