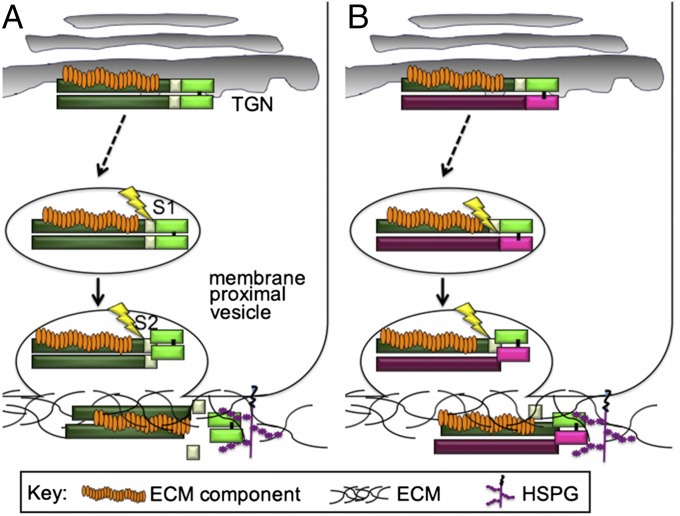
Fig. 6.
Hypothetical model for the role of the prodomain in promoting homodimer and heterodimer activity. (A) One or more ECM proteins bind to the prodomain of BMP4 intracellularly and traffic with the precursor to the cell surface where BMP4 is sequentially cleaved in a membrane proximal compartment. The transient ECM protein/prodomain/mature ligand complex that forms after S1 cleavage ensures that mature BMP4 is deposited in the ECM coincident with S2 cleavage, thereby facilitating association of the homodimeric ligand with HSPGs or other ECM proteins that are required for ligand stability. (B) The BMP4 prodomain, unlike the BMP7 prodomain, is sufficient to enable stable heterodimers to form. Following cleavage, the BMP4 and BMP7 prodomains remain associated with the heterodimeric ligand. The BMP4 prodomain binds to one or more ECM components, thereby anchoring the signaling complex in the matrix and/or presenting the heterodimeric ligand to HSPGs or other binding partners that facilitate signaling.