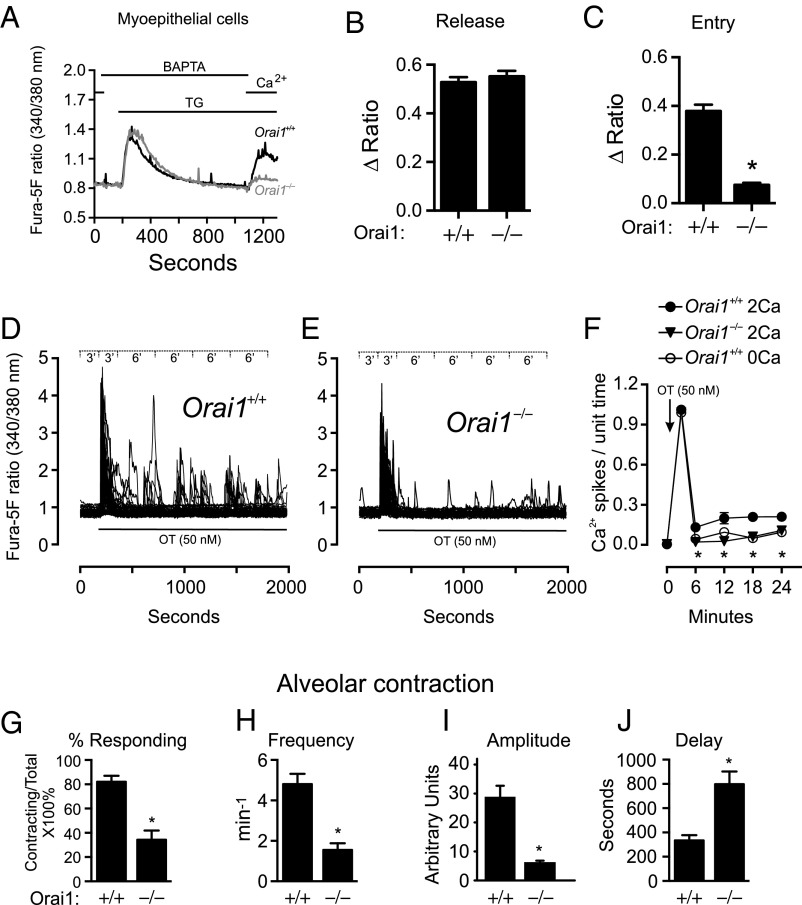
Fig. 4.
Reduced oxytocin-mediated Ca2+ oscillations and alveolar unit contractility in Orai1−/− mice. (A) TG-induced Ca2+ entry in mammary myoepithelial cells isolated from Orai1+/+ (n = 159 cells) and Orai1−/− (n = 128 cells) mice. Cells were bathed in nominally Ca2+-free HBSS supplemented with BAPTA (500 μM) for 2 min and treated with TG (2 μM) to deplete ER Ca2+ stores before readdition of Ca2+ (2 mM). Peak ratio responses to (B) TG (150–270 s) and (C) Ca2+ readdition (1,000–1,180 s). Single-cell ratio responses to oxytocin (50 nM) in (D) Orai1+/+ and (E) Orai1−/− myoepithelial cells loaded with fura-5F; data binning periods are shown in Inset (n = 57 cells). (F) Average number of oxytocin-induced Ca2+ oscillations per data bin in Orai1+/+ myoepithelial cells with 2 mM Ca2+ (filled circle), Orai1−/− myoepithelial cells with 2 mM Ca2+ (filled triangle), and Orai1+/+ myoepithelial cells in the absence of extracellular Ca2+ (open circle) (n = 3 coverslips). Analyses of alveolar unit contractions in live tissue, showing (G) percentage of alveoli responding to oxytocin (50 nM) and (H) frequency, (I) amplitude, and (J) latency of alveolar unit contractions (n = 3 mice). Data represent mean ± SEM; *P < 0.05, Student’s t test (B, C, and G–J) or two-way ANOVA with Bonferroni posttests (F).