Abstract
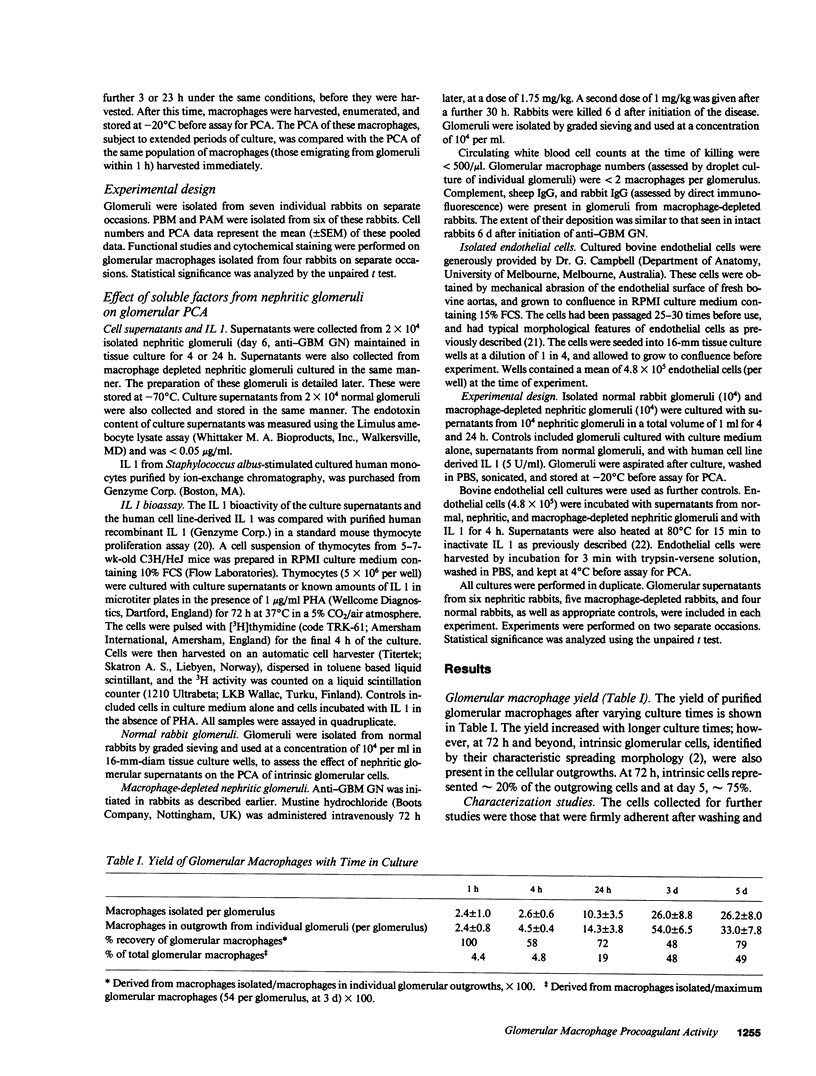
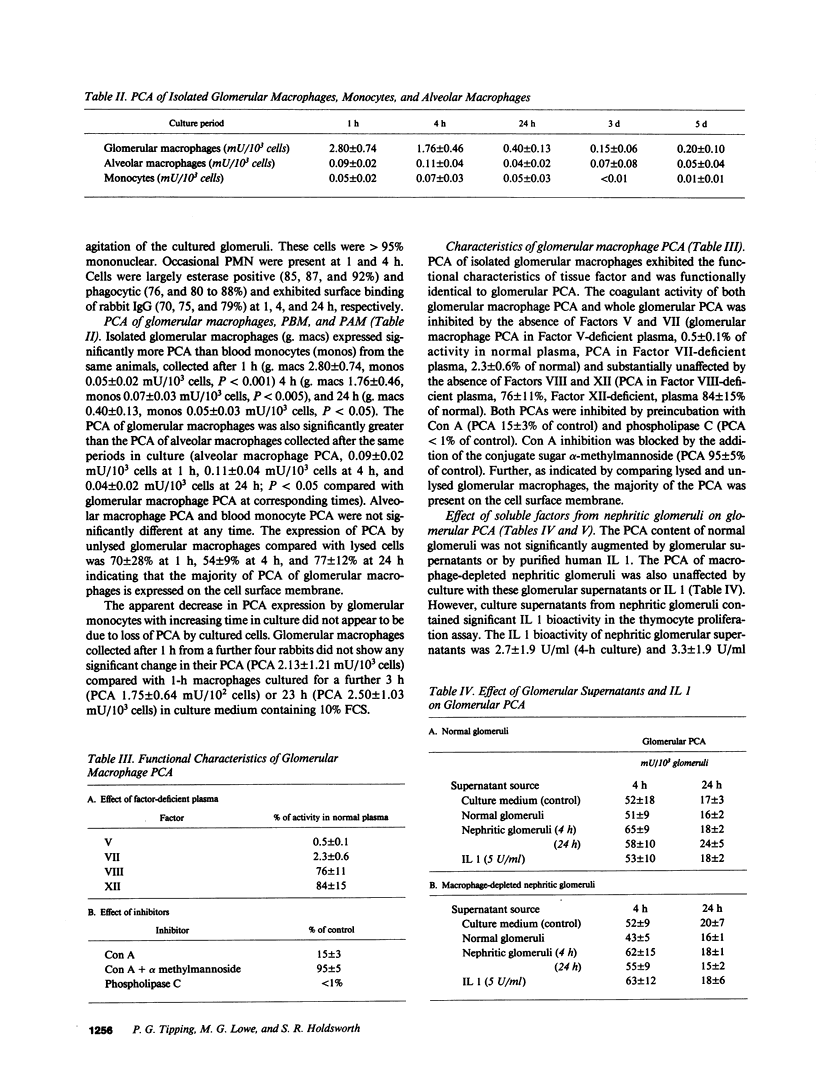
Glomerular fibrin deposition and augmentation of procoagulant activity (PCA) are dependent on glomerular macrophage infiltration in anti-glomerular basement membrane antibody-induced glomerulonephritis (anti-GBM GN) in rabbits. Expression of PCA on the surface of glomerular macrophages and/or augmentation of intrinsic glomerular cell PCA by macrophage cytokines (such as IL 1) are potential mechanisms by which macrophages may augment glomerular PCA. Macrophages were isolated from glomeruli of rabbits developing anti-GBM GN to measure their PCA expression. These macrophages were characterized by morphological and functional criteria. Glomerular macrophages expressed markedly augmented PCA (2.8 +/- 0.7 mU/10(3) cells) compared with blood monocytes (0.05 +/- 0.02 mU/10(3) cells) and alveolar macrophages (0.09 +/- 0.02 mU/10(3) cells) from the same rabbits. Glomerular macrophage PCA was functionally identical to the PCA of whole glomeruli, and was consistent with that of tissue factor. Supernatants from nephritic glomeruli contained IL 1 bioactivity and augmented endothelial cell PCA in vitro. However, these supernatants and purified IL 1 failed to augment the PCA of normal and macrophage-depleted nephritic glomeruli. These studies demonstrate that, in this model of anti-GBM GN, glomerular macrophages contribute directly to the augmented glomerular PCA by their expression of surface membrane PCA, and have the potential to indirectly augment glomerular PCA by their production of cytokines capable of enhancing endothelial cell PCA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevilacqua M. P., Pober J. S., Majeau G. R., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984 Aug 1;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booyse F. M., Sedlak B. J., Rafelson M. E., Jr Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975 Dec 15;34(3):825–839. [PubMed] [Google Scholar]
- Boyce N. W., Tipping P. G., Holdsworth S. R. Lymphokine (MIF) production by glomerular T-lymphocytes in experimental glomerulonephritis. Kidney Int. 1986 Nov;30(5):673–677. doi: 10.1038/ki.1986.239. [DOI] [PubMed] [Google Scholar]
- Edwards R. L., Perla D. The effect of serum on monocyte tissue factor generation. Blood. 1984 Sep;64(3):707–714. [PubMed] [Google Scholar]
- Edwards R. L., Rickles F. R. The role of human T cells (and T cell products) for monocyte tissue factor generation. J Immunol. 1980 Aug;125(2):606–609. [PubMed] [Google Scholar]
- Geczy C. L., Hopper K. E. A mechanism of migration inhibition in delayed-type hypersensitivity reactions. II. Lymphokines promote procoagulant activity of macrophages in vitro. J Immunol. 1981 Mar;126(3):1059–1065. [PubMed] [Google Scholar]
- Hogg N. Human monocytes are associated with the formation of fibrin. J Exp Med. 1983 Feb 1;157(2):473–485. doi: 10.1084/jem.157.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth S. R., Neale T. J., Wilson C. B. The participation of macrophages and monocytes in experimental immune complex glomerulonephritis. Clin Immunol Immunopathol. 1980 Mar;15(3):510–524. doi: 10.1016/0090-1229(80)90063-x. [DOI] [PubMed] [Google Scholar]
- Holdsworth S. R., Thomson N. M., Glasgow E. F., Dowling J. P., Atkins R. C. Tissue culture of isolated glomeruli in experimental crescentic glomerulonephritis. J Exp Med. 1978 Jan 1;147(1):98–109. doi: 10.1084/jem.147.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth S. R., Tipping P. G. Macrophage-induced glomerular fibrin deposition in experimental glomerulonephritis in the rabbit. J Clin Invest. 1985 Oct;76(4):1367–1374. doi: 10.1172/JCI112112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper K. E., Geczy C. L., Davies W. A. A mechanism of migration inhibition in delayed-type hypersensitivity reactions. I. Fibrin deposition on the surface of elicited peritoneal macrophages on vivo. J Immunol. 1981 Mar;126(3):1052–1058. [PubMed] [Google Scholar]
- Hoyer J. R., Michael A. F., Hoyer L. W. Immunofluorescent localization of antihemophilic factor antigen and fibrinogen in human renal diseases. J Clin Invest. 1974 May;53(5):1375–1384. doi: 10.1172/JCI107686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid-Smith P. Coagulation and renal disease. Kidney Int. 1972 Oct;2(4):183–190. doi: 10.1038/ki.1972.93. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Szamel M., Ryan J. L., Sterzel R. B., Gemsa D., Resch K. Interleukin 1 and the glomerular mesangium. I. Purification and characterization of a mesangial cell-derived autogrowth factor. J Immunol. 1986 May 15;136(10):3700–3705. [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Monga G., Mazzucco G., di Belgiojoso G. B., Busnach G. The presence and possible role of monocyte infiltration in human chronic proliferative glomerulonephritides. Light microscopic, immunofluorescence, and histochemical correlations. Am J Pathol. 1979 Feb;94(2):271–284. [PMC free article] [PubMed] [Google Scholar]
- Muhlfelder T. W., Niemetz J., Kreutzer D., Beebe D., Ward P. A., Rosenfeld S. I. C5 chemotactic fragment induces leukocyte production of tissue factor activity: a link between complement and coagulation. J Clin Invest. 1979 Jan;63(1):147–150. doi: 10.1172/JCI109269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986 Mar 1;163(3):740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers R. P., Hathaway W. E., Weston W. L. The endotoxin-induced coagulant activity of human monocytes. Br J Haematol. 1975 Jul;30(3):311–316. doi: 10.1111/j.1365-2141.1975.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Rothberger H., McGee M. P., Lee T. K. Tissue factor activity. A marker of alveolar macrophage maturation in rabbits. Effects of granulomatous pneumonitis. J Clin Invest. 1984 Jun;73(6):1524–1531. doi: 10.1172/JCI111358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberger H., Zimmerman T. S., Spiegelberg H. L., Vaughan J. H. Leukocyte procoagulant activity: enhancement of production in vitro by IgG and antigen-antibody complexes. J Clin Invest. 1977 Mar;59(3):549–557. doi: 10.1172/JCI108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B. S., Levy G. A., Curtiss L. K., Fair D. S., Edgington T. S. Plasma lipoprotein induction and suppression of the generation of cellular procoagulant activity in vitro: two procoagulant activities are produced by peripheral blood mononuclear cells. J Clin Invest. 1981 Jun;67(6):1650–1658. doi: 10.1172/JCI110201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva F. G., Hoyer J. R., Pirani C. L. Sequential studies of glomerular crescent formation in rats with antiglomerular basement membrane-induced glomerulonephritis and the role of coagulation factors. Lab Invest. 1984 Oct;51(4):404–415. [PubMed] [Google Scholar]
- Tipping P. G., Dowling J. P., Holdsworth S. R. Glomerular procoagulant activity in human proliferative glomerulonephritis. J Clin Invest. 1988 Jan;81(1):119–125. doi: 10.1172/JCI113282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping P. G., Holdsworth S. R. The participation of macrophages, glomerular procoagulant activity, and factor VIII in glomerular fibrin deposition. Studies on anti-GBM antibody-induced glomerulonephritis in rabbits. Am J Pathol. 1986 Jul;124(1):10–17. [PMC free article] [PubMed] [Google Scholar]
- Tipping P. G., Neale T. J., Holdsworth S. R. T lymphocyte participation in antibody-induced experimental glomerulonephritis. Kidney Int. 1985 Mar;27(3):530–537. doi: 10.1038/ki.1985.43. [DOI] [PubMed] [Google Scholar]
- Tipping P. G., Worthington L. A., Holdsworth S. R. Quantitation and characterization of glomerular procoagulant activity in experimental glomerulonephritis. Lab Invest. 1987 Feb;56(2):155–159. [PubMed] [Google Scholar]
- Werber H. I., Emancipator S. N., Tykocinski M. L., Sedor J. R. The interleukin 1 gene is expressed by rat glomerular mesangial cells and is augmented in immune complex glomerulonephritis. J Immunol. 1987 May 15;138(10):3207–3212. [PubMed] [Google Scholar]
- Wiggins R. C., Glatfelter A., Brukman J. Procoagulant activity in glomeruli and urine of rabbits with nephrotoxic nephritis. Lab Invest. 1985 Aug;53(2):156–165. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


