Figure 2.
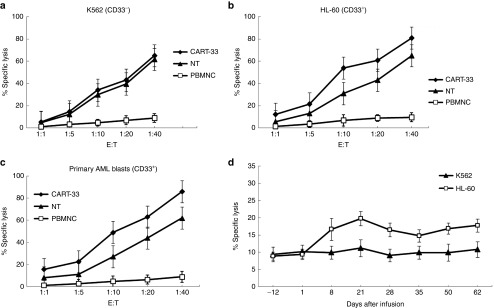
Cytotoxic activity of CART-33 and PBNMC from the patient. Cytotoxic activity of the PBMNC, NT (no transfection T cells) and CART-33 cells obtained from the patient using the following target cells: (a) K562 cell line (human chronic myelogenous leukemia cell lines, CD33−), (b) HL-60 cell line (human promyelocytic leukemia cells, CD33+), and (c) autologous blasts (primary acute myeloid leukemia cells from the patient with CD33+). The results are shown at effector:target (E:T) ratios of 1:1, 5:1, 10:1, 20:1, and 40:1. (d) Cytotoxic activity of the following effector cells obtained from the patient: NT and CART-33 were cultured for 12 days, the PBMNCs obtained from the PB of the patient before and after the CART-33 cell infusion, the target cells were K562 and HL-60, and the results are shown at an E:T ratios of 10:1. The cytotoxic activity was evaluated through a 24-hour carboxyfluorescein succinimidyl ester staining assay. All of the data are represented as the means of triplicate values, and the error bars represent the SEMs. CART, chimeric antigen receptor-modified T cells; PBMNC, peripheral blood mononuclear cell.
