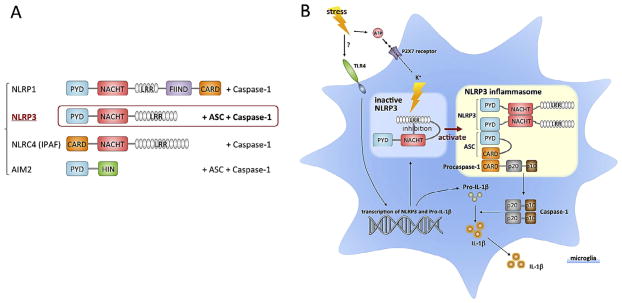
Fig. 2.
Inflammasome subtypes and activation of NLRP3. A. There are four cytosolic pattern recognition receptors that can form inflammasomes that subsequently activate caspase-1 and produce IL-1β. B. The NLRP3 inflammasome undergoes autoinhibition by binding of the LRR domain to the NACHT domain, resulting in blockade of oligomerization and ASC binding. Stimulation relieves this inhibition and enables NLRP3 to bind with pro-caspase-1 through the adaptor protein ASC. Pro-caspase-1 is then cleaved, and the activated caspase-1 cleaves pro-IL-1β, resulting in the release of IL-1β. Interestingly, the NLRP3 inflammasome can detect not only PAMPs, but also DAMPs, which include endogenous danger signals such as ATP. Extracellular ATP binds to the P2X7 receptor ionophore, causing K + efflux, which leads to oligomerization NLRP3. ATP may be associated with stimuli that can cause damage to cells, such as high levels of the excitatory neurotransmitter glutamate, which can cause excitotoxicity. Abbreviations: ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; CARD, caspase-recruitment domain; FIIND, domain with function to find; LRRs, leucine-rich repeats; NACHT, nucleotide-binding oligomerizetion; NLRP3, NLR family, pyrin domain-containing 3; PYD, Pyrin domain.