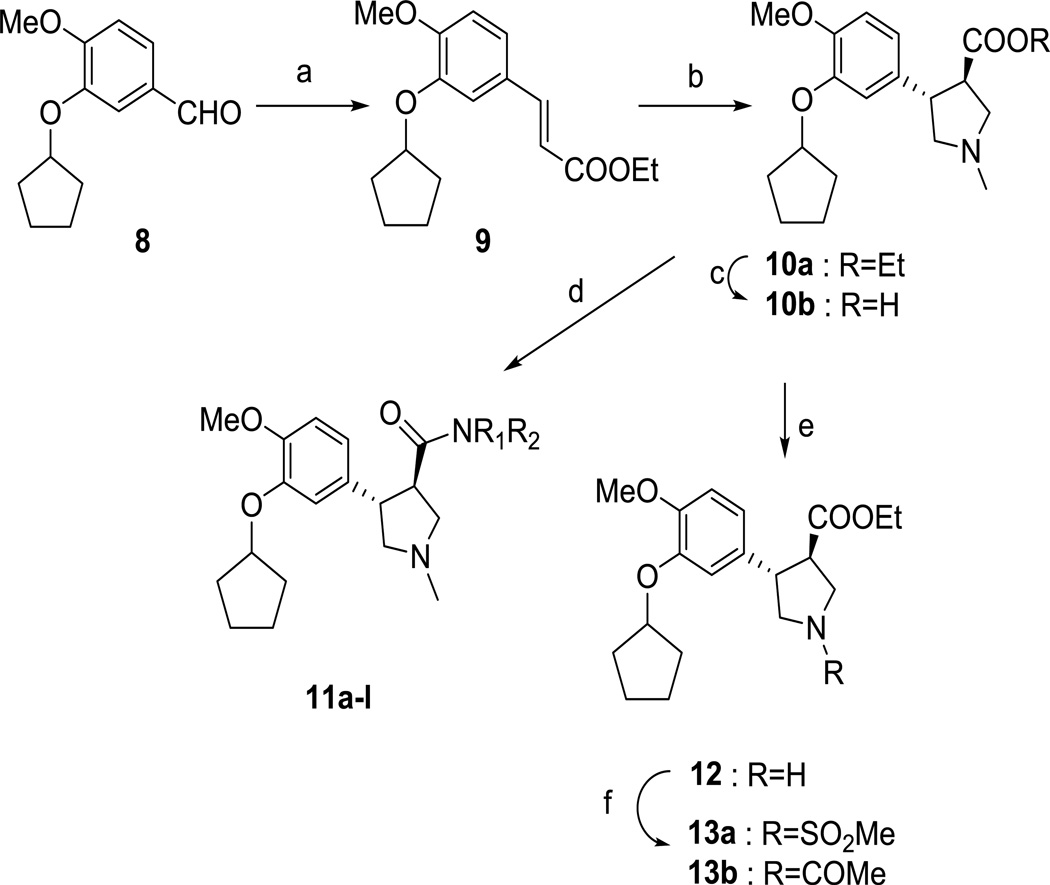
Scheme 1.
Synthesis of pyrrolidine derivatives. Reagents and conditions: (a) (Carbethoxymethylene)triphenylphosphorane, CH3CN, MW, 150 °C, 20 min; (b) formaldehyde, sarcosine, MgSO4, toluene, 170 °C, 24 h; (c) LiOH, H2O, MeOH, THF, rt, 2 h; (d) appropriate amine, Me3Al, Toluene, 80 °C, 12 h; (e) i. 1-Chloroethyl chloroformate, DMAP, 1,2-dichloroethane, reflux, overnight; ii. MeOH, reflux 4h; (f) acetyl chloride or methanesulfonyl chloride, DMAP, DMF, rt, overnight.