Table 1.
rac-(trans-3,4)-Disubstituted pyrrolidine analogs tested against TbrPDEB1
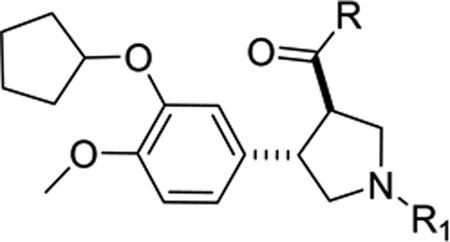 | |||
|---|---|---|---|
| Cpd | R | R1 | TbrPDEB1 (% inh)a |
| 10a | OCH2CH3 | CH3 | 8 ± 11 |
| 10b | OH | CH3 | 17 ± 9 |
| 11a | 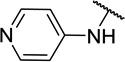 |
CH3 | 5 ± 5c |
| 11b | 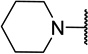 |
CH3 | 7 ± 5c |
| 11c | 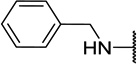 |
CH3 | 8 ± 0 |
| 11d | 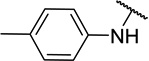 |
CH3 | 6 ± 6 |
| 11e | 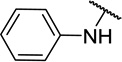 |
CH3 | 16 ± 11 |
| 11f | 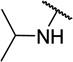 |
CH3 | 10 ± 5 |
| 11g | 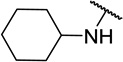 |
CH3 | 16 ± 6 |
| 11h | NHCH3 | CH3 | 8 ± 0 |
| 11i | NHCH2CH3 | CH3 | 5 ± 4 |
| 11j | CH3 | 1 ± 2 | |
| 11k | 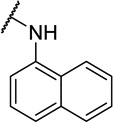 |
CH3 | 10 ± 3 |
| 13a | OCH2CH3 | SO2CH3 | 3 ± 4 |
| 13b | OCH2CH3 | COCH3 | 15 ± 0 |
Data shown are average of 2 replicate independent experiments. Compounds were tested at 10 µM concentrations.
n=1
Replicate of 3 independent experiments.
