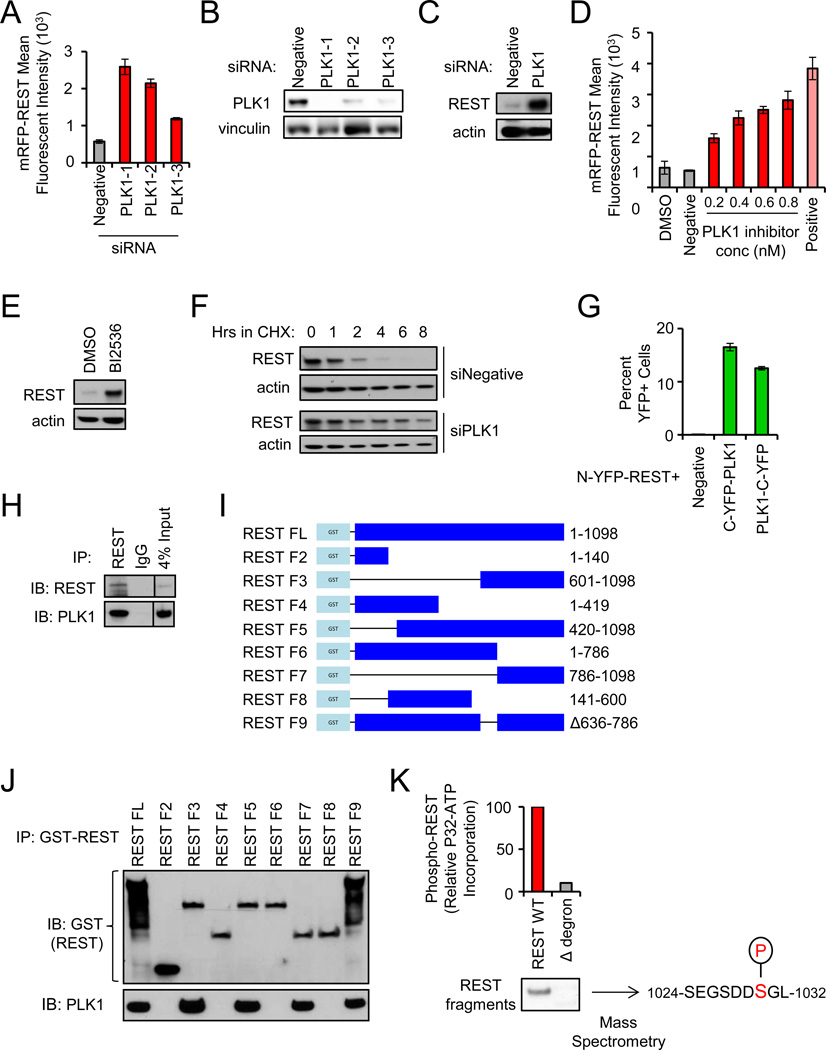
Depletion of PLK1 increases REST abundance. Cells were transfected with the indicated siRNAs and mRFP-REST (n=4). Cellular fluorescence was assessed by flow cytometry. Data presented as mean +/− SE.
Western blot for PLK1 protein levels in cells transfected with the indicated siRNAs.
Depletion of PLK1 increases endogenous REST abundance. 293T cells transfected with the indicated siRNAs were treated with monastrol for 8 hours (to enrich for cells with active PLK1). Endogenous REST protein levels were assessed via western blot.
Pharmacologic inhibition of PLK1 increases REST abundance. 293T cells were treated with BI2536 (PLK1 inhibitor) as shown and transfected with mRFP-REST (n=4). Cellular fluorescence was assessed by flow cytometry. Data presented as mean +/− SE.
Pharmacologic inhibition of PLK1 increases endogenous REST abundance. 293T cells were treated with BI2536 or DMSO. Endogenous REST protein levels were assessed via western blot.
Depletion of PLK1 increases REST protein stability. 293T cells transfected with the indicated siRNAs were treated with cycloheximide and analyzed for REST protein levels.
PLK1 interacts with REST. HMECs expressing N-YFP-REST were transduced with C-YFP-PLK1 or PLK1-C-YFP retroviruses. Cellular fluorescence was assessed by flow cytometry. Data presented as mean +/− SE.
Endogenous REST was immunoprecipitated from monastrol-treated 293T cells and analyzed for REST and PLK1 protein by western blot.
GST-REST fragments used for interaction studies. Numbers indicate amino acids included in each fragment.
PLK1 interacts with the C-terminus of REST. 293T cells were transfected with the GST-REST fragments from I. After MG132 treatment (to inhibit proteasome function), interaction was assessed by GST pull down and western blot for GST (top) and PLK1 (bottom).
REST is phosphorylated by PLK1 on serine-1030. Purified REST protein fragments (wild type or Δdegron) were incubated with active PLK1 and ATP [γ32P]. Audioradiography and mass spectrometry were performed. The serine within the REST phospho-degron phosphorylated by PLK1 is highlighted.