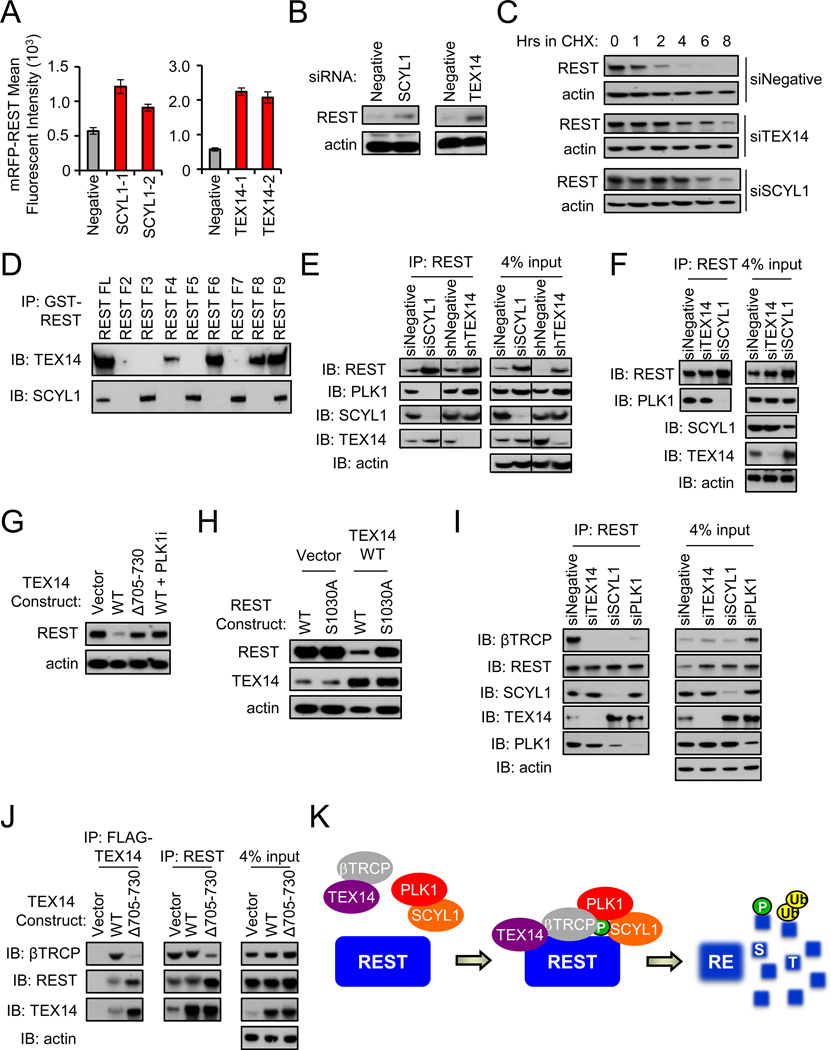
Depletion of SCYL1 and TEX14 increases REST abundance. Cells were transfected with the indicated siRNAs and mRFP-REST (n=4). Cellular fluorescence was assessed by flow cytometry. Data presented as mean +/− SE.
Depletion of SCYL1 and TEX14 increases endogenous REST abundance. 293T cells transfected with the indicated siRNAs were treated with monastrol and endogenous REST protein was assessed via western blot.
Depletion of SCYL1 and TEX14 increases REST protein stability. 293T cells transfected with the indicated siRNA were treated with cycloheximide for as shown. REST protein levels were assessed by western blot.
SCYL1 interacts with the C-terminus of REST while TEX14 interacts with the N-terminus of REST. 293T cells were transfected with the GST-REST fragments (
Fig 2I). After MG132 treatment, interaction was assessed by GST pull down and western blot.
SCYL1 is required for the PLK1-REST interaction. Endogenous REST was immunoprecipitated from monastrol-treated 293T cells transfected with the indicated siRNAs and analyzed via western blot.
SCYL1 is required for the PLK1-REST interaction in human TNBC cells. BT549 TNBC cells were treated with MLN4924 (to inhibit REST-ubiquitination) and transfected with the indicated siRNAs. Endogenous REST was immunoprecipitated and analyzed via western blot.
TEX14-mediated regulation of REST abundance requires TEX14 amino acids 705–730. 293T cells were transfected with the indicated FLAG-TEX14 constructs and treated with the BI2536. Western blot analysis was performed for REST and actin (loading control).
TEX14 regulates REST abundance through the PLK1-phosphorylated REST-degron. 293T cells were transfected with the indicated expression vectors and treated with monastrol. Western blot analysis was performed for REST, TEX14, and actin (loading control).
TEX14 is required for the REST-βTRCP interaction. Endogenous REST was immunoprecipitated from MLN4924-treated 293T cells transfected with the indicated siRNAs and analyzed via western blot.
TEX14 links REST and βTRCP. MLN4924-treated 293T cells were transfected with the indicated FLAG-TEX14 constructs. FLAG-TEX14 or endogenous REST was immunoprecipitated and analyzed via western blot analysis.
Model of REST regulation by the STP axis. The STP axis (SCYL1-TEX14-PLK1) cooperates to regulate REST phosphorylation and βTRCP recruitment. REST is subsequently targeted for degradation by SCFβTRCP.