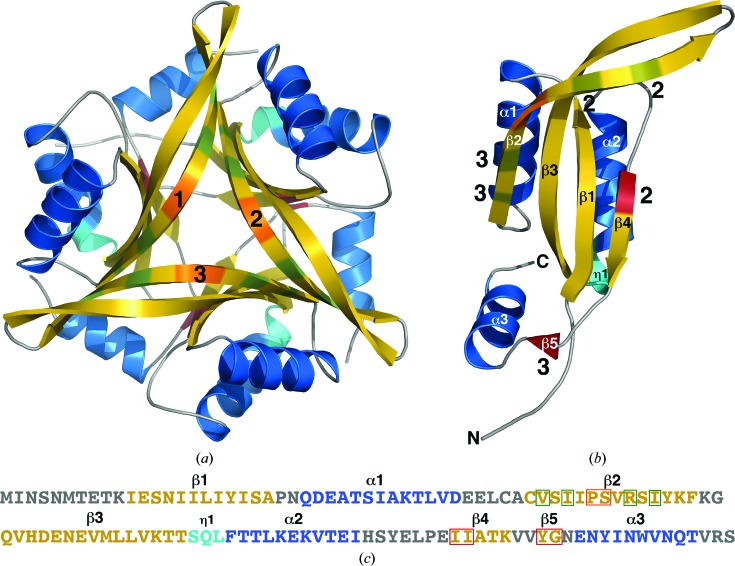
Figure 1.
(a) Cartoon representations of the trimer (a) and single protomer (b) in the crystal structure of Cp-CutA1 (PDB entry 4e98). The β-strands are colored gold, α-helices blue and 310-helices (η1) cyan, and the protomer chains are numbered 1–3. Two types of intertwined hydrogen bonds between the three protomers help to stabilize the trimeric structure. One type, colored green, involves dual interprotomer antiparallel hydrogen bonds between opposing residues in β2. The other type, colored firebrick red, involves single interprotomer antiparallel hydrogen bonds between opposing residues in β4 and β5. In chain 1 (b) the interprotomer positions of these intertwined hydrogen bonds are indicated by the numbers 2 and 3. The wide β-bulge in β2 is colored orange. (c) The primary amino acid sequence of Cp-CutA1 with the elements of secondary structure colored similar to the cartoon representations. Residues participating in the two types of interprotomer hydrogen bonds are highlighted in a green or red box and the residues of the β-bulge are highlighted in an orange box.