Abstract
Antigen-specific immunotherapy aims to selectively restore tolerance to innocuous antigens in cases of autoimmune or allergic disease, without the need for general immune suppression. Although the principle of antigen-specific immunotherapy was discovered more than a century ago, its clinical application to date is limited, particularly in the control of autoimmunity. This has resulted mainly from a lack of in-depth understanding of the underlying mechanism. More recently, the differentiation of extra-thymically induced T regulatory (Treg) cell subsets has been shown to be instrumental in peripheral tolerance induction. Two main types of inducible Treg cells, interleukin-10-secreting or Foxp3+, have now been described, each with distinct characteristics and methods of therapeutic induction. It is crucial, therefore, to identify the suitability of either subset in the control of specific immune disorders. This review explores their natural function, the known mechanisms of therapeutic differentiation of either subset as well as their in vivo functionality and discusses new developments that may aid their use in antigen-specific immunotherapy, with a focus on autoimmune disease.
Keywords: antigen specificity, Foxp3, interleukin-10, immunotherapy, regulatory T cell
Introduction
Aberrant activation of the immune system can lead to autoimmune disease or allergy. Commonly, these conditions are treated with general immune-modulating substances which, although often highly effective at treating the primary symptoms, frequently lead to adverse effects. By now more than a century has passed since Leonard Noon first discovered that therapeutic administration of the causative antigen of an immune disturbance can educate the immune system and restore a healthy response to the antigen, without affecting general immune function. More recent advances have elucidated that therapeutically induced tolerance involves a range of immunological changes, including the de novo differentiation of extra-thymically inducible CD4+ T-cell receptor-αβ (TCR-αβ) T regulatory (Treg) cell subsets.
Identification and classification of inducible Treg cells
So far, two broad subsets of inducible Treg cells have been identified; interleukin-10 (IL-10) -secreting, Foxp3– Treg cells [hereafter referred to as IL-10Treg cells but sometimes also called type 1 regulatory (Tr1) cells], and peripherally induced Foxp3+ Treg cells. Inducible Treg cells are widely recognized as being important for homoeostatic or therapeutically induced T-cell tolerance, yet the lack of specific markers for either subset has complicated the study of their in vivo differentiation and function. For example, co-expression of CD49b and LAG-3 has been reported to specifically identify a population of IL-10Treg cells both in man and mouse.1 However, more recent findings from a study of antigen-specific immunotherapy in a murine model of autoimmune disease suggest that these markers are not specific identifiers of all IL-10Treg cells, with co-expression found on a fraction of IL-10Treg cells but also on other T cells that do not express IL-10.2 Moreover, to distinguish peripherally differentiated Foxp3+ Treg (pTreg) cells from resident thymus-derived Foxp3+ Treg (tTreg) cells, two main differentiating markers, Helios (present on murine and human tTreg but not pTreg cells) and Neuropilin-1 (present only on murine tTreg cells), have been reported, but again neither are undisputed.3–7 This lack of exclusive markers has limited the ability to track and study inducible Treg cells in vivo. In the case of Foxp3+ Treg cells in particular, much of our current understanding results from studies using TCR-transgenic, Rag-deficient mice that lack endogenous Foxp3 expression or from in vitro differentiated Treg (iTreg) cells, which are similar but not necessarily identical to in vivo differentiated pTreg cells, phenotypically and functionally.8,9 Of course the latter is likely to be the case when comparing in vitro or in vivo differentiated IL-10Treg cells as well, although here no formal distinction in nomenclature is made. Despite these shortcomings, we will endeavour to review here the known pros and cons of both subtypes of inducible Treg cell, how to generate them, and their suitability as targets in antigen-specific immunotherapy (ASIT).
The natural role of inducible Treg cells in immune regulation
To understand the therapeutic potential of inducible Treg cell subsets, it is important to first understand the natural development and function of these cells in the prevention of disease. The first in vivo demonstration of the regulatory role of IL-10Treg cells was in patients with severe combined immunodeficiency who received HLA-mismatched haematopoietic stem cell transplants, where donor T cells expressed high quantities of IL-10 and were responsible for tolerance to host antigens.10 A role for IL-10Treg cells in maintaining immune homoeostasis to gut flora in mice was suggested after the discovery of their presence in the intestinal lamina propria.11 In 2004, Akdis et al.12 first clearly demonstrated a natural role for IL-10Treg cells in maintaining a healthy immune balance in humans by revealing that, in comparison to allergy sufferers; healthy individuals harbour a greater frequency of IL-10-secreting rather than interferon-γ-secreting or IL-4-secreting CD4+ T cells specific for common environmental antigens. This study was later followed up by demonstrating that, in healthy individuals, the frequency of allergen-specific IL-10Treg cells among CD4+ T cells increases with higher exposure to the antigen.13 Akin to the allergy study, the Peakman group demonstrated that in healthy individuals the T-cell response to islet antigens shows a bias towards IL-10, in contrast to diabetes patients who exhibited polarization towards a T helper type 1 (Th1) response.14,15 In multiple sclerosis, IL-10Treg cells from patients demonstrated a reduction in IL-10 secretion, associated with a reduced suppressive ability.16,17 Finally, IL-10Treg cells were shown to curtail collateral damage caused by enduring immune responses to chronic infection.18 Differentiation of IL-10Treg cells from chronically activated effector T cells therefore seems a generally conserved negative feedback mechanism.
Experimental animal models suggest that, similar to IL-10Treg cells, Foxp3+ pTreg cells are important for the induction and maintenance of mucosal tolerance. Three independent groups reported simultaneously that pTreg cells are generated in the intestine under the influence of the vitamin A metabolite all-trans retinoic acid, secreted by mucosal dendritic cells.19–21 In addition, short-chain fatty acids produced by commensal microorganisms in mice were shown to promote extra-thymic Foxp3 induction in CD4+ T cells that mediate an anti-inflammatory response.22,23 In addition to mucosal sites, pTreg cells can develop within other peripheral tissues. In a murine model of uveoretinitis, tissue-resident and locally differentiated pTreg cells protected from retinal damage.24 The pTreg cells have also been reported to develop in response to chronic inflammation resulting from asthma, autoimmune disease or infection and therefore appear to play a role in limiting the tissue damage that inevitably results from long-lasting inflammation, although these findings are not universally supported (as reviewed thoroughly by Bilate and Lafaille25). Interestingly, comparison of various animal models of autoimmune disease, each carrying the same modified version of Foxp3 protein that affects the development of pTreg cells but not tTreg cells, suggests that pTreg cells play a pivotal role in preventing the onset of type 1 diabetes but not arthritis or autoimmune encephalomyelitis.26–28 Disease-specific conditions therefore seem to play an important role in the functionality of pTreg cells.
Clearly, both subsets of inducible Treg cells fulfil a diverse natural role in immune homoeostasis and both seem potent, albeit not universal, inhibitors of undesirable immune responses. This supports the notion that the therapeutic differentiation of these T cells should be a prime aim for immunotherapy of hyperimmune conditions.
Immune regulation by inducible Treg cells; a pivotal role for IL-10
Several mechanisms have been reported for the suppressive function of IL-10Treg cells and inducible Foxp3+ Treg cells. These include cell contact-dependent negative co-stimulatory molecules including cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), programmed death-1 (PD-1), lymphocyte activation gene-3 (LAG-3) and inducible T-cell costimulator (ICOS) and surface molecules that mediate metabolic disruption such as CD39 and CD73.29 It is becoming increasingly clear, however, that the immunosuppressive cytokine IL-10 plays a vital role in mediating the function of not only IL-10Treg cells but also Foxp3+ Treg cells. Interleukin-10 has recently been shown to be important not only for Foxp3+ Treg cell-mediated in vitro suppression30 but also for in vivo regulation in models of colitis31 and central nervous system (CNS) autoimmune disease32. Interleukin-10 is produced by a wide variety of other immune cells, yet CD4+ T-cell-derived IL-10 has a dampening effect on the immune response either by directly affecting other T cells or through the regulation of dendritic cell function.33 It not only suppresses T cells that may otherwise exert undesirable immune responses, but appears to actively promote further differentiation and stability of IL-10Treg cells and Foxp3+ Treg cells,31,34,35 although IL-10 is not required for initial in vitro differentiation of Foxp3+ iTreg cells.32 Despite being a common mediator of suppression for both types of inducible Treg cells, IL-10 on its own has not proven a suitable candidate for immunotherapy as clinical trials with the administration of exogenous IL-10 have shown limited benefit and considerable adverse effects.36 Clearly, a cellular source of this pleiotropic cytokine is required to direct adequate delivery for immune regulation.
In vitro differentiation of inducible Treg cells for immunotherapy
To harness their therapeutic potential, many laboratories have developed methods for the in vitro differentiation of inducible Treg cells, with the ultimate aim of achieving immune regulation by adoptive transfer.
In vitro, inducible Treg cells can be differentiated and expanded efficiently. The differentiation of IL-10Treg cells was first described after chronic activation of naive CD4+ T cells in the presence of IL-10.37 A similar subset of IL-10Treg cells can be produced by stimulating human T cells with antibodies against CD3 and the co-stimulatory molecule CD46 in the presence of IL-2.38 Furthermore, both human and murine IL-10Treg cells can be produced by activation of CD4+ T cells in the presence of vitamin D3 and dexamethasone.39 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), which binds the ligand-activated transcription factor aryl hydrocarbon receptor, also promotes the induction of IL-10 expression in human naive CD4+ T cells upon activation.40 It is important to note, though, that none of these methods generate a pure population of IL-10-secreting T cells. Generally, not more than 50% of the differentiated cells express IL-10, whereas both IL-10+ and IL-10– retain the ability to produce effector cytokines. The addition of neutralizing antibodies to IL-12, interferon-γ and IL-4 during differentiation does, however, abrogate this.39
In vitro differentiation of Foxp3+ iTreg cells at very high purity (> 90%) can be achieved by activating naive CD4+ T cells with anti-CD3 and anti-CD28 in the presence of transforming growth factor-β and high doses of IL-2.3 Differentiation of iTreg cells using antigen and antigen-presenting cells, however, typically does not give rise to a population more than 75% Foxp3+, even if using naive TCR-transgenic CD4+ T cells.28 Several agents have now been demonstrated to augment Foxp3 expression and improve in vivo functionality upon adoptive transfer of iTreg cells. As expected from its natural role in the gut, all-trans retinoic acid was shown to promote iTreg cell differentiation. It is debated, however, whether this results from a direct effect on signalling downstream of cytokine receptors41,42 or is an indirect result of relieving the inhibitory effect of pre-activated/memory T cells in the culture.43 According to a recent study, retinoic acid promotes the suppressive capacity and stability of iTreg cells upon in vivo transfer in a skin allograft model.44 Similar to IL-10Treg cells, the aryl hydrocarbon receptor plays a role in the differentiation of Foxp3+ iTreg cells. Whether the aryl hydrocarbon receptor promotes the development of IL-10Treg cells, Foxp3+ iTreg cells or indeed potentially pathogenic Th17 cells depends on a combination of the cytokines in the culture and the nature of the specific ligand.40,45,46 PD-L1, the ligand for the co-inhibitory receptor programmed death 1, has been shown to promote the induction of Foxp3 expression in human and murine CD4+ T cells.47–50 This suggests that modulation of the PD-1–PD-L1 axis could be used to amplify conversion of naive T cells into iTreg cells, although a recent study using PD-1 knock-out mice found that PD-1 was non-essential for Foxp3 induction.51 Other, less well-defined methods of promoting Foxp3 induction that have been reported recently include the use of rapamycin,52 blocking antibody to the adhesion molecule leukocyte function-associated antigen-1,53 the growth factor progranulin,54 the glucocorticoid-induced leucine zipper protein,55 the Notch ligand Delta-like 1 (DL1 or DLL1),56 depletion of essential amino acids,57 or drugs that prevent the proteolysis of the transcription factor Krüppel-like factor 258 (see also Table1). Finally, changing common cell culture conditions to a hypoxic environment has been suggested to improve Foxp3 induction in vitro.59
Table 1.
Factors that promote inducible regulatory T (Treg) cell differentiation upon activation, in vitro
| IL-10 Treg cells | Foxp3+ iTreg cells | ||
|---|---|---|---|
| Exogenous IL-10 | Groux et al.37 | Retinoic acid | Xiao et al.41, Mucida et al.42, Hill et al.43 |
| CD46 ligation | Kemper et al.38 | Aryl hydrocarbon receptor ligation | Gandhi et al.40, Quintana et al.45, Mezrich et al.46 |
| Vitamin D3 and dexamethosone | Barrat et al.39 | PD-1–PD-L1 interaction | Wang et al.47, Francisco et al.48, Amarnath et al.49, Chen et al.50 |
| Aryl hydrocarbon receptor ligation | Gandhi et al.40 | Rapamycin | Hippen et al.52 |
| Blockade of leukocyte function-associated antigen-1 | Verhagen et al.53 | ||
| Progranulin | Wei et al.54 | ||
| Glucocorticoid-induced leucine zipper (GILZ) | Bereshchenko et al.55 | ||
| Delta-like 1 mediated Notch signalling | Mota et al.56 | ||
| Inhibition of Krüppel-like factor 2 (KLF2) | Pabbisetty et al.58 | ||
| Depletion of essential amino acids | Cobbold et al.57 | ||
Efficacy of immunotherapy based on the transfer of ex vivo differentiated inducible Treg cells
Pre-clinical studies have revealed that adoptive transfer of CD4+ Treg cells can provide effective immune suppression. For example, transferring ex vivo expanded Foxp3+ tTreg cells can suppress inhibitory antibody formation in haemophilia,60 delay allograft rejection61 and protect against autoimmune cholangitis62 and rheumatoid arthritis.63 Focusing on Treg cells differentiated in vitro, adoptive transfer of IL-10Treg cells has been shown to protect against colitis,11,37 rheumatoid arthritis64 and CNS autoimmune disease,39 whereas Foxp3+ iTreg cells have been shown to suppress colitis,31 graft rejection,65 spontaneous abortion,66 graft-versus-host disease52 and CNS autoimmune disease (Table2).32,67,68 This success has led to the translation of Treg cell therapy to the clinic, with several trials using adoptive transfer of ex vivo expanded thymic Treg cells or in vitro differentiated extra-thymic Treg cells to treat autoimmune disease, transplant rejection or graft-versus-host disease recently completed, currently underway or recruiting patients (see clinicaltrials.gov and Table2). The first results have been promising and appear to show some efficacy and indicate that the principle of Treg cell therapy is safe.69–73 Crucially, however, an increasing number of studies have shown that antigen-specificity improves Treg cell functionality, regardless of the subset of interest, and reduces the risk of off-target immunosuppressive effects.30,32,39,74–76 It is important to note, though, that simply inducing the expression of a regulatory factor like Foxp3 in antigen-specific T cells does not necessarily produce a suppressive phenotype.77 Although it is possible to differentiate antigen-specific extra-thymic Treg cells in vitro before adoptive transfer, it is challenging to obtain significant cell numbers at high purity and also costly to provide a bespoke treatment for every patient. In addition, concerns have been raised about the stability of the phenotype of in vitro differentiated Foxp3+ iTreg cells in particular, following reports that these may revert to a pathogenic phenotype.63 These concerns and others suggest that in vivo differentiation of inducible Treg cells may be preferable over transfer of ex vivo differentiated cells, although both methods have their own advantages and challenges (Fig.1).
Table 2.
Pre-clinical disease models and early stage clinical trials (Italics) showing efficacy and/or safety of regulatory T (Treg) cell transfer
| Expanded thymic Foxp3+ Treg cells | Foxp3+ iTreg cells | IL-10Treg cells | |
|---|---|---|---|
| Colitis | ✓ 31 | ✓ 11,37 | |
| Rheumatoid arthritis | ✓ 63 | ✓ 64 | |
| Central nervous system autoimmune disease | ✓ 32,67,68 | ✓ 39 | |
| Graft-versus-host disease | ✓ 69–71 | ✓ 52 | ✓ 73 |
| Graft rejection | ✓ 61,65 | ✓ 65 | |
| Antibody formation in haemophilia | ✓ 60 | ||
| Autoimmune cholangitis | ✓ 62 | ||
| Spontaneous abortion | ✓ 66 | ||
| Crohn's disease | ✓ 72 |
Figure 1.
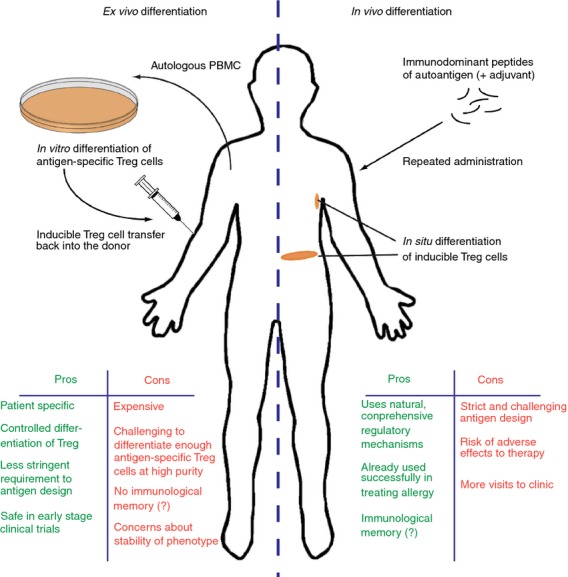
Transfer versus in situ differentiation of inducible regulatory T (Treg) cells in antigen-specific immunotherapy of autoimmune disease. Two different antigen-mediated treatment strategies aimed at using the therapeutic potential of inducible Treg cells are considered for immunotherapy; ex vivo differentiation of autologous CD4+ T cells followed by transfer back into the donor or in situ differentiation by administration of tolerogenic peptide, either alone or in combination with a tolerogenic adjuvant.
Antigen-mediated differentiation of inducible Treg cells in situ
It has long been known that immune tolerance, associated with the differentiation of inducible Treg cells, can be achieved by therapeutic administration of relevant antigen.75 Importantly, it is here that we find a fundamental difference between the differentiation of IL-10Treg cells and Foxp3+ pTreg cells.
The therapeutic differentiation of IL-10Treg cells in vivo is the best-established method of ASIT and generally mimics the natural differentiation of such cells, as described above for allergen-specific cells.13 As shown in our laboratory and by others, the induction of IL-10Treg cells by ASIT requires the repeated exposure to high doses of specific antigen (reviewed by Ng et al. and Sabatos-Peyton et al.33,75). Expression of IL-10 is up-regulated in pre-differentiated Th1, Th2 as well as Th17 cells upon repeated activation, which demonstrates that this negative feedback mechanism is applicable to dampening the immune response in a wide range of conditions. The requirement for high doses of antigen carries an inherent risk of adverse effects, particularly in patients who already demonstrate an undesirable immune response to the relevant antigen. Therefore, before applying ASIT to target the induction of IL-10Treg cells, careful considerations ought to be made regarding the route of administration and dosing strategy. We have shown previously, in a murine model, that intranasal administration of myelin basic protein-derived peptide provides tolerance and protection from CNS autoimmune disease without severe adverse effects.78 In the clinic, intranasal administration is less practical because the dose of antigen administered is relatively difficult to control. Recently, we demonstrated that the same peptide can be used safely for subcutaneous tolerization, provided that a dose escalation protocol is followed.2 Analysis of the CD4+ T-cell transcriptome during the dose escalation protocol revealed progressive suppression of pro-inflammatory mediators and repression of the cell cycle pathway, coinciding with up-regulation of IL-10 and co-inhibitory receptors. With this knowledge, it may now be easier to find suitable adjuvants that, in combination with specific antigen, can be used to obtain the desired phenotype for IL-10Treg cells more efficiently, while also further reducing the risk of undesirable immune activation and adverse effects. It has been shown already that targeting antigen uptake by dendritic cells via a scavenger receptor favours specifically the differentiation of IL-10Treg cells.79 In a similar vein, antigen coupled to either autologous apoptotic cells or, perhaps more elegantly, synthetic biodegradable microparticles, which promotes antigen uptake via scavenger receptors, has been shown to promote immune tolerance, although a clear role for IL-10Treg cells in this system has yet to be defined.80–82 It further remains to be elucidated if other factors that promote the differentiation of IL-10Treg cells in vitro, such as ligation of aryl hydrocarbon receptor40 or rapamycin and anti-CD45RB83, may be used in combination with antigen-specific therapy to augment IL-10 production in vivo.
Both IL-10Treg cells and Foxp3+ pTreg cells develop in response to chronic antigen encounter, but whereas the development of IL-10Treg cells requires high antigen doses, Foxp3+ pTreg cells develop in response to very low levels of antigen,84,85 whereas strong TCR signals actively prevent Foxp3 expression.86 Several methods of antigen administration have been demonstrated to give rise to pTreg cell differentiation, including subcutaneous infusion,84 targeting of the antigen to dendritic cells using DEC-205,85 oral administration87 and ectopic expression of antigen in the liver.88,89 In an interesting novel approach, mice received systemic sublethal irradiation to induce apoptosis of immune cells before antigen administration.90 This approach was shown to improve Foxp3+ pTreg cell differentiation and antigen-specific tolerance in models of multiple sclerosis and diabetes but, importantly, without affecting the antibacterial response. Many of these methods take advantage of either naturally high or therapeutically enhanced levels of transforming growth factor-β at the site of treatment, which promotes pTreg cell development.
Whereas IL-10Treg cells may develop from differentiated effector T cells, Foxp3+ pTreg cells are generally considered to develop from naive T cells only. This requirement for a naive T-cell phenotype has discouraged some researchers from attempting in vivo differentiation of Foxp3+ pTreg cells considering that, in the clinic, ASIT will inevitably follow the onset of disease, meaning that effector/memory CD4+ T cells will be present. However, even during inflammation, not all T cells in the body that are specific for relevant antigens will be activated. From unpublished personal observations we would conclude that although pre-activated T cells impair Foxp3 induction in naive T cells, the conversion is not fully abrogated. It remains to be elucidated whether the remaining conversion suffices for immune suppression in vivo. Excitingly, several of the adjuvants described to promote the (in vitro) differentiation of inducible Foxp3+ Treg cells have been reported to either relieve the suppressive effect of memory T cells43 and inflammatory conditions54 on the induction of Foxp3 expression in naive CD4+ T cells or even augment the conversion of pre-activated/memory CD4+ T cells56. In situ differentiation of either subset of inducible Treg cells seems a feasible approach, provided that an optimized protocol is applied.
Functionality and phenotypic stability of inducible Treg cells in vivo
In dose escalation immunotherapy, IL-10Treg cell-mediated tolerance could be induced after antigen priming and provided long-lasting homoeostatic protection.2 The allergy beekeeper model has demonstrated that the frequency of IL-10Treg cells correlates with the level of antigenic exposure and that a high level of antigen is required for an enduring response dominated by IL-10.13 Although the number of IL-10Treg cells diminishes in the absence of specific antigen, this is rapidly restored upon subsequent encounter with high levels of antigen.
The question of whether Foxp3+ pTreg cells can be differentiated and are functional as well as stable in an inflammatory setting is highly contentious. Probably, this is due to a range of factors, including the general inability to distinguish pTreg cells from tTreg cells, the fact that most of the data available are achieved using iTreg cells rather than pTreg cells, and the broad heterogeneity in the specificity and phenotype of Treg cell populations used. First, although some groups have reported the differentiation of pTreg cells in response to chronic inflammation, so suggesting that they provide a negative feedback mechanism similar to that offered by IL-10Treg cells, this was not observed by others.25 Moreover, several groups have reported that chronic activation of inducible Foxp3+ Treg cells with specific antigen and the presence of pro-inflammatory cytokines such as IL-6 and tumour necrosis factor-α can impair the stability of the regulatory phenotype and lead to the conversion of Treg cells to pathogenic Th1 or Th17 cells.91–94 In direct contrast, tumour necrosis factor receptor 2 was shown to be critical for Treg cell stability in a colitis model,95 whereas in a model of CNS autoimmune disease IL-6 was not only found to be ineffective in converting Treg cells into Th17 cells,96 it also abrogated granulocyte–macrophage colony-stimulating factor production in iTreg cells specifically and thereby suppressed pathogenic conversion.97 Similarly, murine iTreg cells have been reported to retain their suppressive ability under Th1-polarizing conditions,98 whereas a study on Foxp3+ Treg cells from patients with relapsing–remitting multiple sclerosis demonstrated that IL-12 promoted interferon-γ secretion and reduced suppressive function.99 These conflicting results indicate that the differentiation, functionality and phenotypic stability of pTreg cells, under inflammatory conditions, may vary greatly depending on the origin of cells that gave rise to them, the method used for their differentiation and the disease-specific conditions. It seems clear that there is a degree of plasticity in the phenotypic stability of Foxp3+ pTreg cells, but it remains to be elucidated if this plasticity has a negative impact on their suitability as a target for immunotherapy or if, just like IL-10Treg cells, pTreg cells can retain a memory of suppressive function, as proposed as part of the recently coined ‘revised heterogeneity model’.100
Requirements of antigen suitable for Treg cell differentiation in vivo
As mentioned earlier, Foxp3+ pTreg cell differentiation can be achieved through the administration of sub-immunogenic levels of antigen. Despite this, a strong binding of the peptide to MHC II seems to be required. The Von Boehmer group reported that only a mimotope for the insulin B:9–23 peptide with increased MHC affinity but not the natural peptide induced the differentiation of pTreg cells and protected from diabetes in NOD mice.101 This is in line with the finding that T cells of high antigen affinity are more readily converted into Foxp3+ Treg cells compared with T cells that recognize the same antigen with lower affinity.102 In our own studies of the efficacy of IL-10Treg cell and Foxp3+ iTreg cell differentiation and their suppressive function in vivo, we revealed a direct correlation between MHC II affinity of variants of the immunodominant myelin basic protein peptide Ac1-9 and IL-10Treg cell formation,103 but were able to generate functional Foxp3+ iTreg cells using the lower affinity variant, in vitro.28 However, although subcutaneous administration of the low-affinity peptide variant alone promotes the development of Foxp3+ pTreg cells in vivo, we have yet to achieve protection from CNS autoimmune disease with this approach (J. Verhagen, unpublished observation). Although further study of the role of antigen affinity is required, these results emphasize that epitope selection forms a crucial step in the design of ASIT. This is the case particularly in autoimmune disease where CD4+ T cells that recognize auto-antigens of high MHC affinity will mostly have been deleted during thymic selection and as a result immunodominant epitopes responsible for pathology are often of relatively low affinity. The use of altered peptide ligands to treat autoimmune disease is controversial after complications following high-dose administration of peptide antigen with augmented TCR affinity in a phase 2 trial in multiple sclerosis.104,105 This, however, should not occur when targeting the induction of pTreg cells instead of IL-10Treg cells, as no adverse effects were observed at lower doses. Moreover, this effect is unlikely to occur with peptide of altered MHC affinity rather than altered TCR affinity.2 Nevertheless, the alteration of peptide affinity may not be required for successful antigen-specific differentiation of Treg cells. As mentioned above, an increasing number of adjuvants have been reported that may aid the development of IL-10Treg cells or Foxp3+ pTreg cells by modifying the activatory signals through the TCR or co-factors such as co-stimulatory molecules, cytokine receptors or adhesion molecules. The adjuvants could allow for tolerance induction with peptides of lower MHC affinity. In addition, it remains critical for the success of ASIT of many autoimmune diseases to further identify suitable immunodominant epitopes. We have already discussed that in several autoimmune settings inducible Treg cells have been reported to be important for a healthy homoeostatic balance. It is currently unclear if autoimmune disease results primarily from a defect in central or peripheral tolerance, but from the importance of inducible Treg cells in homoeostasis we can conclude that natural self-antigens of an affinity sufficient to induce extra-thymic Treg cell differentiation do exist. One might hypothesize that immunotherapy based on Treg cell differentiation would benefit most from promoting tolerance to antigens that are of an affinity that falls within the, as yet undefined, range that makes them naturally susceptible to regulation by inducible Treg cells. To extrapolate this even further, one might argue that, based on their natural role, differentiation of IL-10Treg cells should be more suitable for tolerance induction to abundant antigen (e.g. proteolipid protein or myelin basic protein in the case of myelin sheath antigens involved in CNS autoimmune disease). On the other hand, pTreg cell differentiation may better suit rarer antigens (such as myelin oligodendrocyte glycoprotein). An unequivocal method of distinguishing IL-10Treg cells, pTreg cells and tTreg cells in combination with novel single cell omics analysis may reveal if indeed tolerance to individual antigens within the same tissue is regulated by distinct subtypes of Treg cells. Furthermore, if a clear division of labour as hypothesized exists, it will be important for the efficacy of immunotherapy to demonstrate if both IL-10Treg cells and Foxp3+ pTreg cells can exert bystander suppression. For example, can IL-10Treg cells specific for protein A suppress immune responses to the related protein B, even if protein B is normally regulated by Foxp3+ pTreg cells or vice versa?
Interaction between Foxp3+ pTreg cells and IL-10Treg cells
So far, we have considered whether it would be advantageous to target the differentiation of either IL-10Treg cells or Foxp3+ pTreg cells. However, the greatest success for safe and enduring tolerance induction using ASIT may not rely on the choice of the preferred subset, but rather on inducing the differentiation of both. After all, both subsets have been demonstrated to have a wide range of specificity and the ability to suppress Th1-, Th2- and Th17-dominated disease. Furthermore, although the nature of the cells that can convert into either subtype as well as the quantity of antigen required may vary greatly, the nature of the optimal antigens themselves seems comparable for both IL-10Treg cells and Foxp3+ pTreg cells. This dichotomy in peripheral T-cell regulation does indeed seem to occur. Depending on environmental signals, both IL-10Treg cells and Foxp3+ pTreg cells were found to be involved in regulating the immune response to the same antigen during fungal infection.106 Similarly, in a model of transplant tolerance both IL-10Treg cells and Foxp3+ Treg cells were found to play diverse and non-redundant roles during long-term immune regulation, each in distinct physiological sites.107 These authors found that while Foxp3+ Treg cells initiated tolerance in their model, IL-10Treg cells provided enduring protection. This is reminiscent of our own findings in dose-escalation immunotherapy, where the initial, low, doses of antigen triggered the accumulation of Foxp3+ T cells, whereas the higher doses needed for enduring tolerance induced IL-10Treg cell differentiation.2 In this model, however, we have yet to confirm if the surge in Foxp3+ Treg cells at the early stages of tolerization results from de novo differentiation of Foxp3+ pTreg cells or relative expansion of tTreg cells. In any case, it seems reasonable that given the right antigen, both IL-10Treg cells and Foxp3+ pTreg cells may differentiate simultaneously at distinct sites considering that both develop under different environmental conditions (Fig.2). A recent comprehensive analysis of human T-cell compartmentalization elegantly demonstrated that the distribution of naive, effector and memory T cells throughout the human body varies greatly from site to site.108 This, combined with variations in the level of antigen exposure and natural variation in environmental factors (e.g. transforming growth factor-β) at various physiological sites, strongly supports a devolved mechanism of tolerance.
Figure 2.
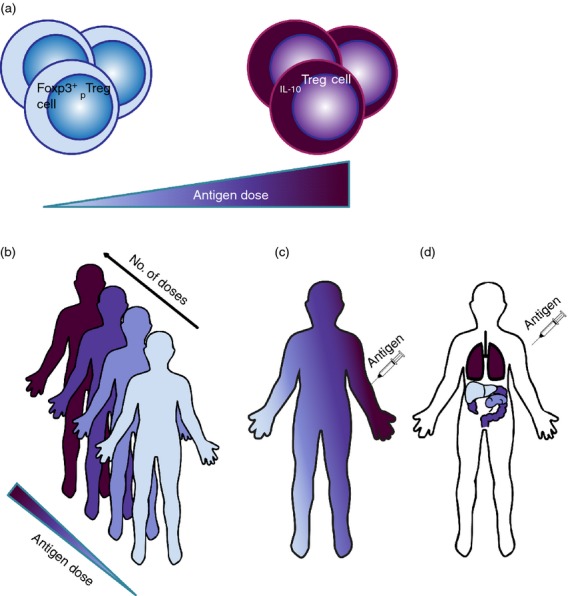
Theoretical correlation between Foxp3+ peripherally induced regulatory T (pTreg) cell and interleukin-10-secreting Treg (IL-10Treg) cell formation in response to therapeutic antigen. (a) Quantity of antigen forms a crucial decider in determining whether Foxp3+ pTreg cells or IL-10Treg cells are formed. This may affect the appearance and distribution of inducible Treg cells in several ways. (b) In dose escalation immunotherapy, the early low doses appear to favour Fox3+ pTreg cell formation, whereas the later high doses promote IL-10Treg cell differentiation. It remains to be elucidated whether there is a causal or functional link between the two. (c) Proximity to the site of injection, and therefore the level of antigen exposure, may determine if IL-10Treg cells or Foxp3+ pTreg cells are formed. (d) IL-10Treg and Foxp3+ pTreg cells may develop simultaneously after antigen administration but in distinct physiological sites (chosen at will for this illustration). This would depend on antigen penetrance, but also on the local variety of antigen-presenting cells and/or the local cytokine environment (e.g. presence of transforming growth factor-β).
Conclusions
Novel insights increasingly support the notion that peripheral regulation plays a crucial role in the maintenance of autoimmune homeostasis. Improved understanding of inducible Treg cell differentiation and function allows for the development of more refined approaches to ASIT by advancing the design of therapeutic peptide and the use of adjuvants to augment inducible Treg cell conversion. Although the first results of immunotherapy trials based on Treg cell transfer have been promising, we feel that in situ differentiation of inducible Treg cells remains the optimal strategy for the induction of efficacious and enduring immune regulation in autoimmune disease. With the arrival of the omics era we will be able to improve our understanding of the spatiotemporal contribution of inducible Treg cell subsets in tolerance induction. This should aid the further development of dosing strategies, including the optimal quantity and route of administration. The exciting developments in this field promise to propel the development of immunotherapeutic strategies and will hopefully lead to ASIT of autoimmune disease finally accomplishing its promise of a wide-scale treatment of unprecedented specificity and efficacy.
Acknowledgments
JV and DCW are supported by Wellcome Trust programme grant 091074. AW receives a grant from the Marie Curie Initial Training Networks (NeuroKine).
Glossary
Abbreviations:
- ASIT
antigen-specific immunotherapy
- CNS
central nervous system
- IL-10
interleukin-10
- IL-10Treg cell
interleukin-10-secreting T regulatory cell
- PD-1
programmed cell death protein 1
- iTreg cell
in vitro-induced T regulatory cell
- pTreg cell
peripherally-induced T regulatory cell
- TCR
T-cell receptor
- Th1
T helper type 1
- tTreg
thymic T regulatory cell
Disclosures
The authors declare no financial or commercial conflict of interest.
References
- Gagliani N, Magnani CF, Huber S, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19:739–46. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- Burton BR, Britton GJ, Fang H, et al. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nat Commun. 2014;5:4741. doi: 10.1038/ncomms5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen J, Wraith DC. Comment on “Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells”. J Immunol. 2010;185:7129. doi: 10.4049/jimmunol.1090105. ; author reply 30. [DOI] [PubMed] [Google Scholar]
- Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6:e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Louvet C, Davini D, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–22. doi: 10.1084/jem.20120822. S1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Bilate AM, Gobert M, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723–42. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas AK, Benoist C, Bluestone JA, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14:307–8. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014;259:88–102. doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetta R, Bigler M, Touraine JL, et al. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y, Weaver CT, Lazenby A, Elson CO. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol. 2002;169:6112–9. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- Akdis M, Verhagen J, Taylor A, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205:2887–98. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif S, Tree TI, Astill TP, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004;113:451–63. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree TI, Lawson J, Edwards H, Skowera A, Arif S, Roep BO, Peakman M. Naturally arising human CD4 T-cells that recognize islet autoantigens and secrete interleukin-10 regulate proinflammatory T-cell responses via linked suppression. Diabetes. 2010;59:1451–60. doi: 10.2337/db09-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116:3252–7. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Forero I, Garcia-Munoz R, Martinez-Pasamar S, et al. IL-10 suppressor activity and ex vivo Tr1 cell function are impaired in multiple sclerosis. Eur J Immunol. 2008;38:576–86. doi: 10.1002/eji.200737271. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med. 2007;204:239–43. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson SW, Heuss ND, Gregerson DS. Local “on-demand” generation and function of antigen-specific Foxp3+ regulatory T cells. J Immunol. 2013;190:4971–81. doi: 10.4049/jimmunol.1202625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–58. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- Bettini ML, Pan F, Bettini M, et al. Loss of epigenetic modification driven by the Foxp3 transcription factor leads to regulatory T cell insufficiency. Immunity. 2012;36:717–30. doi: 10.1016/j.immuni.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darce J, Rudra D, Li L, et al. An N-terminal mutation of the Foxp3 transcription factor alleviates arthritis but exacerbates diabetes. Immunity. 2012;36:731–41. doi: 10.1016/j.immuni.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen J, Burton BR, Britton GJ, Shepard ER, Anderton SM, Wraith DC. Modification of the FoxP3 transcription factor principally affects inducible T regulatory cells in a model of experimental autoimmune encephalomyelitis. PLoS One. 2013;8:e61334. doi: 10.1371/journal.pone.0061334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori S, Goudy KS, Roncarolo MG. The cellular and molecular mechanisms of immuno-suppression by human type 1 regulatory T cells. Front Immunol. 2012;3:30. doi: 10.3389/fimmu.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay G, Shevach EM. Antigen-specific induced T regulatory cells impair dendritic cell function via an IL-10/MARCH1-dependent mechanism. J Immunol. 2013;191:5875–84. doi: 10.4049/jimmunol.1301693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt EG, Haribhai D, Williams JB, et al. IL-10 produced by induced regulatory T cells (iTregs) controls colitis and pathogenic ex-iTregs during immunotherapy. J Immunol. 2012;189:5638–48. doi: 10.4049/jimmunol.1200936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen J, Gabrysova L, Shepard ER, Wraith DC. CTLA-4 modulates the differentiation of inducible Foxp3+ Treg cells but IL-10 mediates their function in experimental autoimmune encephalomyelitis. PLoS One. 2014;9:e108023. doi: 10.1371/journal.pone.0108023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol. 2013;4:129. doi: 10.3389/fimmu.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25– cells to develop suppressive activity: the role of IL-2, TGF-β, and IL-10. J Immunol. 2004;172:5213–21. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–84. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A, Khosraviani S, Noel S, Mohan D, Donner T, Hamad AR. Interleukin-10 paradox: a potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine. 2014 doi: 10.1016/j.cyto.2014.10.031. ; doi: 10.1016/j.cyto.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–92. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi R, Kumar D, Burns EJ, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3+ regulatory T cells. Nat Immunol. 2010;11:846–53. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-β-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–84. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Pino-Lagos K, Kim G, et al. Retinoic acid can directly promote TGF-β-mediated Foxp3+ Treg cell conversion of naive T cells. Immunity. 2009;30:471–2. doi: 10.1016/j.immuni.2009.03.008. ; author reply 2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Hall JA, Sun CM, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi cells. Immunity. 2008;29:758–70. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Tejon G, Fuentes C, et al. Alloreactive regulatory T cells generated with retinoic acid prevent skin allograft rejection. Eur J Immunol. 2015;45:452–63. doi: 10.1002/eji.201444743. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–8. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:9331–6. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarnath S, Mangus CW, Wang JC, et al. The PDL1–PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3:111ra20. doi: 10.1126/scitranslmed.3003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Fosco D, Kline DE, Meng L, Nishi S, Savage PA, Kline J. PD-1 regulates extrathymic regulatory T-cell differentiation. Eur J Immunol. 2014;44:2603–16. doi: 10.1002/eji.201344423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellestad KK, Thangavelu G, Ewen CL, Boon L, Anderson CC. PD-1 is not required for natural or peripherally induced regulatory T cells: severe autoimmunity despite normal production of regulatory T cells. Eur J Immunol. 2014;44:3560–72. doi: 10.1002/eji.201444688. [DOI] [PubMed] [Google Scholar]
- Hippen KL, Merkel SC, Schirm DK, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11:1148–57. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen J, Wraith DC. Blockade of LFA-1 augments in vitro differentiation of antigen-induced Foxp3+ Treg cells. J Immunol Methods. 2014;414:58–64. doi: 10.1016/j.jim.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Zhang Y, Zhao W, Yu X, Liu CJ. Progranulin facilitates conversion and function of regulatory T cells under inflammatory conditions. PLoS One. 2014;9:e112110. doi: 10.1371/journal.pone.0112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereshchenko O, Coppo M, Bruscoli S, et al. GILZ promotes production of peripherally induced Treg cells and mediates the crosstalk between glucocorticoids and TGF-β signaling. Cell Rep. 2014;7:464–75. doi: 10.1016/j.celrep.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Mota C, Nunes-Silva V, Pires AR, Matoso P, Victorino RM, Sousa AE, Caramalho I. Delta-like 1-mediated notch signaling enhances the in vitro conversion of human memory CD4 T cells into FOXP3-expressing regulatory T cells. J Immunol. 2014;193:5854–62. doi: 10.4049/jimmunol.1400198. [DOI] [PubMed] [Google Scholar]
- Cobbold SP, Adams E, Farquhar CA, et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci USA. 2009;106:12055–60. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabbisetty SK, Rabacal W, Maseda D, et al. KLF2 is a rate-limiting transcription factor that can be targeted to enhance regulatory T-cell production. Proc Natl Acad Sci USA. 2014;111:9579–84. doi: 10.1073/pnas.1323493111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neildez-Nguyen TM, Bigot J, Da Rocha S, Corre G, Boisgerault F, Paldi A, Galy A. Hypoxic culture conditions enhance the generation of regulatory T cells. Immunology. 2014;144:431–43. doi: 10.1111/imm.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Biswas M, Liao G, et al. Ex vivo expanded autologous polyclonal regulatory T cells suppress inhibitor formation in hemophilia. Mol Ther Methods Clin Dev. 2014;1:14030. doi: 10.1038/mtm.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Ma L, Zhao M, et al. Ex vivo expanded human regulatory T cells delay islet allograft rejection via inhibiting islet-derived monocyte chemoattractant protein-1 production in CD34+ stem cells-reconstituted NOD-scid IL2rγnull mice. PLoS One. 2014;9:e90387. doi: 10.1371/journal.pone.0090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Zhang W, Yang GX, et al. Successful immunotherapy of autoimmune cholangitis by adoptive transfer of forkhead box protein 3+ regulatory T cells. Clin Exp Immunol. 2014;178:253–61. doi: 10.1111/cei.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti M, Spreafico R, Saidin S, et al. Ex vivo-expanded but not in vitro-induced human regulatory T cells are candidates for cell therapy in autoimmune diseases thanks to stable demethylation of the FOXP3 regulatory T cell-specific demethylated region. J Immunol. 2015;194:113–24. doi: 10.4049/jimmunol.1401145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnagli H, Martire D, Belmonte N, et al. Type 1 regulatory T cells specific for collagen type II as an efficient cell-based therapy in arthritis. Arthritis Res Ther. 2014;16:R115. doi: 10.1186/ar4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasato F, Morita R, Schichita T, et al. Prevention of allogeneic cardiac graft rejection by transfer of ex vivo expanded antigen-specific regulatory T-cells. PLoS One. 2014;9:e87722. doi: 10.1371/journal.pone.0087722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Liu X, Shan B, Wu J, Sharma S, Sun Y. Prevention of CpG-induced pregnancy disruption by adoptive transfer of in vitro-induced regulatory T cells. PLoS One. 2014;9:e94702. doi: 10.1371/journal.pone.0094702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj RK, Geiger TL. Mitigation of experimental allergic encephalomyelitis by TGF-β induced Foxp3+ regulatory T lymphocytes through the induction of anergy and infectious tolerance. J Immunol. 2008;180:2830–8. doi: 10.4049/jimmunol.180.5.2830. [DOI] [PubMed] [Google Scholar]
- Zhang H, Podojil JR, Chang J, Luo X, Miller SD. TGF-β-induced myelin peptide-specific regulatory T cells mediate antigen-specific suppression of induction of experimental autoimmune encephalomyelitis. J Immunol. 2010;184:6629–36. doi: 10.4049/jimmunol.0904044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzonkowski P, Bieniaszewska M, Juscinska J, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127– T regulatory cells. Clin Immunol. 2009;133:22–6. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–70. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–8. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- Desreumaux P, Foussat A, Allez M, et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn's disease. Gastroenterology. 2012;143:1207–17. doi: 10.1053/j.gastro.2012.07.116. e1–2. [DOI] [PubMed] [Google Scholar]
- Bacchetta R, Lucarelli B, Sartirana C, et al. Immunological outcome in haploidentical-HSC transplanted patients treated with IL-10-anergized donor T cells. Front Immunol. 2014;5:16. doi: 10.3389/fimmu.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011;3:83ra42. doi: 10.1126/scitranslmed.3002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatos-Peyton CA, Verhagen J, Wraith DC. Antigen-specific immunotherapy of autoimmune and allergic diseases. Curr Opin Immunol. 2010;22:609–15. doi: 10.1016/j.coi.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RP, Jabri B. Vaccine against autoimmune disease: antigen-specific immunotherapy. Curr Opin Immunol. 2013;25:410–7. doi: 10.1016/j.coi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarikonda G, Fousteri G, Sachithanantham S, et al. BDC12-4.1 T-cell receptor transgenic insulin-specific CD4 T cells are resistant to in vitro differentiation into functional Foxp3+ T regulatory cells. PLoS One. 2014;9:e112242. doi: 10.1371/journal.pone.0112242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrysova L, Nicolson KS, Streeter HB, Verhagen J, Sabatos-Peyton CA, Morgan DJ, Wraith DC. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells. J Exp Med. 2009;206:1755–67. doi: 10.1084/jem.20082118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Romain G, Flamar AL, et al. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J Exp Med. 2012;209:109–21. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts DR, McCarthy DP, Miller SD. Exploiting apoptosis for therapeutic tolerance induction. J Immunol. 2013;191:5341–6. doi: 10.4049/jimmunol.1302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts DR, Martin AJ, McCarthy DP, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30:1217–24. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado RA, LaMothe RA, Ferrari JD, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci USA. 2015;112:E156–65. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Gregori S, Jofra T, et al. Rapamycin combined with anti-CD45RB mAb and IL-10 or with G-CSF induces tolerance in a stringent mouse model of islet transplantation. PLoS One. 2011;6:e28434. doi: 10.1371/journal.pone.0028434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–8. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- Molinero LL, Miller ML, Evaristo C, Alegre ML. High TCR stimuli prevent induced regulatory T cell differentiation in a NF-κB-dependent manner. J Immunol. 2011;186:4609–17. doi: 10.4049/jimmunol.1002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–33. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luth S, Huber S, Schramm C, et al. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J Clin Invest. 2008;118:3403–10. doi: 10.1172/JCI32132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carambia A, Freund B, Schwinge D, et al. TGF-β-dependent induction of CD4+CD25+Foxp3+ Tregs by liver sinusoidal endothelial cells. J Hepatol. 2014;61:594–9. doi: 10.1016/j.jhep.2014.04.027. [DOI] [PubMed] [Google Scholar]
- Kasagi S, Zhang P, Che L, et al. In vivo-generated antigen-specific regulatory T cells treat autoimmunity without compromising antibacterial immune response. Sci Transl Med. 2014;6:241ra78. doi: 10.1126/scitranslmed.3008895. [DOI] [PubMed] [Google Scholar]
- Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity. 2013;39:949–62. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence A, Amarnath S, Mariotti J, et al. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity. 2012;37:209–22. doi: 10.1016/j.immuni.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Zheng Y, Li R, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat Med. 2013;19:322–8. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- Komatsu N, Okamoto K, Sawa S, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62–8. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- Chen X, Wu X, Zhou Q, Howard OM, Netea MG, Oppenheim JJ. TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. J Immunol. 2013;190:1076–84. doi: 10.4049/jimmunol.1202659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor RA, Floess S, Huehn J, Jones SA, Anderton SM. Foxp3+ Treg cells in the inflamed CNS are insensitive to IL-6-driven IL-17 production. Eur J Immunol. 2012;42:1174–9. doi: 10.1002/eji.201142216. [DOI] [PubMed] [Google Scholar]
- Reynolds BC, Turner DG, McPherson RC, et al. Exposure to inflammatory cytokines selectively limits GM-CSF production by induced T regulatory cells. Eur J Immunol. 2014;44:3342–52. doi: 10.1002/eji.201444687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor RA, Leech MD, Suffner J, Hammerling GJ, Anderton SM. Myelin-reactive, TGF-β-induced regulatory T cells can be programmed to develop Th1-like effector function but remain less proinflammatory than myelin-reactive Th1 effectors and can suppress pathogenic T cell clonal expansion in vivo. J Immunol. 2010;185:7235–43. doi: 10.4049/jimmunol.1001551. [DOI] [PubMed] [Google Scholar]
- Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–5. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S. Lineage stability and phenotypic plasticity of Foxp3+ regulatory T cells. Immunol Rev. 2014;259:159–72. doi: 10.1111/imr.12175. [DOI] [PubMed] [Google Scholar]
- Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J Exp Med. 2011;208:1501–10. doi: 10.1084/jem.20110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Auffray C, Delpoux A, et al. Highly self-reactive naive CD4 T cells are prone to differentiate into regulatory T cells. Nat Commun. 2013;4:2209. doi: 10.1038/ncomms3209. [DOI] [PubMed] [Google Scholar]
- Gabrysova L, Wraith DC. Antigenic strength controls the generation of antigen-specific IL-10-secreting T regulatory cells. Eur J Immunol. 2010;40:1386–95. doi: 10.1002/eji.200940151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B, Goodwin B, Richert N, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–75. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, Steinman L. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The altered peptide ligand in relapsing MS Study Group. Nat Med. 2000;6:1176–82. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]
- Bedke T, Iannitti RG, De Luca A, et al. Distinct and complementary roles for Aspergillus fumigatus-specific Tr1 and Foxp3+ regulatory T cells in humans and mice. Immunol Cell Biol. 2014;92:659–70. doi: 10.1038/icb.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Jofra T, Valle A, et al. Transplant tolerance to pancreatic islets is initiated in the graft and sustained in the spleen. Am J Transplant. 2013;13:1963–75. doi: 10.1111/ajt.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome JJ, Yudanin N, Ohmura Y, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159:814–28. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]


