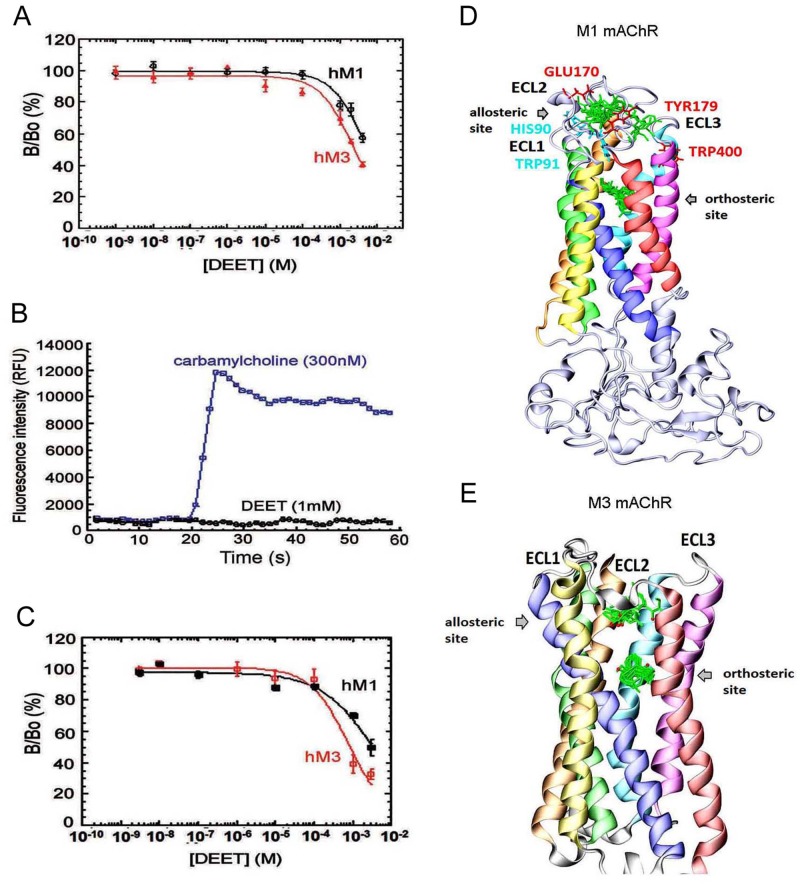
Fig 4. DEET interacts with mammal M1 and M3 muscarinic receptors.
The functional properties of DEET are investigated using CHO cells expressing human M1 (hM1) and M3 (hM3) mAChR subtypes. A) Dose-dependent inhibition of [3H]-NMS binding to M1 and M3 human muscarinic ACh receptor (mAChR) subtypes by DEET. The results are expressed as the ratio of the specific [3H]-NMS binding measured with (B) or without DEET (Bo). B-C) Signals acquired for calcium fluorescence after the addition at 20 sec of carbamylcholine (CCh) (300nM) and DEET (1mM) on CHO-hM1 cells (n = 3). DEET inhibition of the Ca2+ mobilization after pretreatment of the cells with increasing concentrations of DEET (3nM to 3 mM), followed by a sub-maximal concentration of CCh (100 nM) (C). D-E) in silico Docking of DEET into human M1 and rat M3 mAChRs. The two binding regions (allosteric and orthosteric sites) of DEET molecules (green) in human M1 mAChR are represented (D). In red are shown residues of a M1 mAChR monomer interacting with MT-7 loops previously indentified. Analogous interaction residues from the second M1 mAChR monomer are indicated in blue (D) close to the hypothetical M1 mAChR allosteric site occupied by DEET. The in silico docking results to rat M3 mAChR crystal structure 4DAJ (E) also show that DEET binds on two distinct sites (allosteric and orthosteric sites). Ten DEET poses from each group located in both sites are shown in green. The following colour coding for transmembrane helices used: TM1—orange, TM2—green, TM3—dark blue, TM4—yellow, TM5—red, TM6—magenta, TM7—light blue. ECL, extracellular loop.