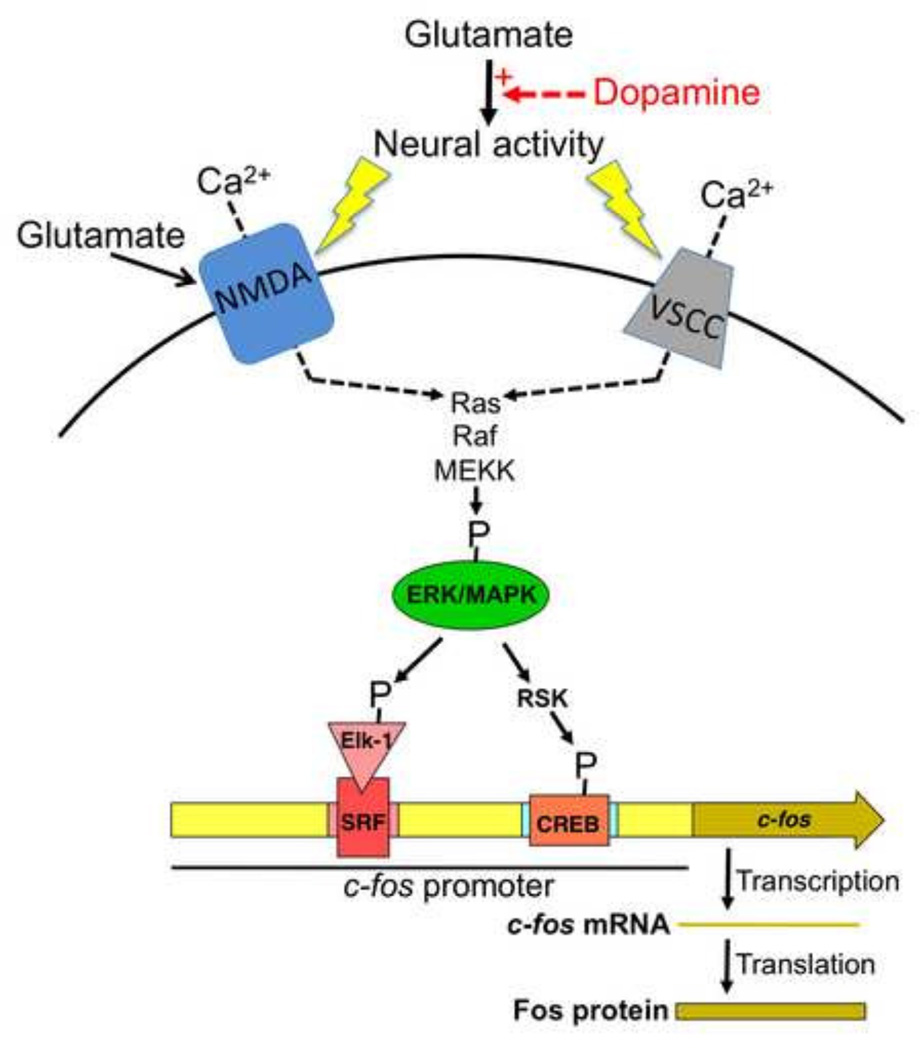
Figure 2.
Schematic drawing to illustrate the neurochemistry and molecular mechanisms of c-fos and Fos expression in strongly activated neurons in prefrontal cortex and nucleus accumbens of awake behaving animals. Glutamate is the main excitatory neurotransmitter that increases neural activity. Dopamine enhances (red arrow and + sign) glutamate-mediated neural activation of the small proportion of neurons that have the highest levels of neural activity while inhibiting neural activation of the majority of neurons that have lower levels of neural activity. The detailed electrophysiological mechanisms underlying this glutamate-dopamine interaction are described in (Surmeier et al., 2007). Strong persistent neural activity induces calcium (Ca2+) influx through NMDA-type glutamate receptors and voltage-sensitive calcium channels (VSCCs) to levels that are sufficient for phosphorylating and activating ERK/MAPK via the Ras-Raf-MEKK pathway. ERK/MAPK activation leads to phosphorylation of Elk-1 that is associated with serum response factor (SRF) as well as phosphorylation of CREB via ribosomal S6 kinase (RSK). Elk-1/SRF and CREB are transcription factors that, when phosphorylated, can induce transcription of the coding sequence for c-fos. Transcribed c-fos mRNA and the translated protein product Fos can be used as markers of strongly activated neurons.