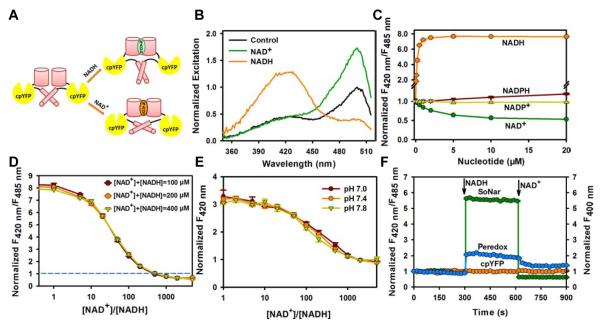
Figure 1. Genetically encoded sensor for NAD+, NADH, and their ratio.
(A) Design of SoNar, which is a fusion of cpYFP and the NADH-binding domain of T-Rex. Binding of NAD+ or NADH both induces changes in protein conformation and fluorescence. (B) Excitation spectra of purified SoNar in the control condition (black), and after addition of 20 μM NAD+ (green) or 20 μM NADH (orange), normalized to the peak intensity in the control condition. Emission was measured at 530 nm. (C) Normalized ratio of fluorescence intensities excited at 420 nm and 485 nm (F420 nm/F485 nm) in the presence of different concentrations of NADH and its analogs. (D) Fluorescence ratios plotted against the NAD+/NADH ratio at the indicated total nicotinamide adenine dinucleotide concentration. Fluorescence ratios were normalized to the control condition in the absence of nucleotides. (E) Fluorescence excited at 420 nm plotted against the NAD+/NADH ratio at the indicated pH. Fluorescence was normalized to the control condition in the absence of pyridine nucleotides at pH 7.4. (F) Kinetics of fluorescence response of purified SoNar, Peredox, and cpYFP protein to sequential addition of 0.2 μM NADH and 2 mM NAD+. (C-F), Error bars represent SEM. See also Figure S1 and Table S1.