Abstract
Background
Inadequate intrapleural drug concentrations caused by poor penetration of systemic antibiotics into the pleural cavity is a major cause of treatment failure in empyema. Herein, we describe a novel antibiotic-eluting pigtail catheter coated with electrospun nanofibers used for the sustained release of bactericidal concentrations of penicillin in the pleural space.
Methods
Electrospun nanofibers prepared using polylactide-polyglycolide copolymer and penicillin G sodium dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol were used to coat the surface of an Fr6 pigtail catheter. The in vitro patterns of drug release were tested by placing the catheter in phosphate-buffered saline. In vivo studies were performed using rabbits treated with penicillin either intrapleurally (Group 1, 20 mg delivered through the catheter) or systemically (Group 2, intramuscular injection, 10 mg/kg). Penicillin concentrations in the serum and pleural fluid were then measured and compared.
Results
In vitro studies revealed a burst release of penicillin (10% of the total dose) occurring in the first 24 hours, followed by a sustained release in the subsequent 30 days. Intrapleural drug levels were significantly higher in Group 1 than in Group 2 (P<0.001). In the former, penicillin concentrations remained above the minimum inhibitory concentration breakpoint throughout the entire study period. In contrast, serum penicillin levels were significantly higher in Group 2 than in Group 1 (P<0.001). Notably, all Group 2 rabbits showed signs of systemic toxicity (paralytic ileus and weight loss).
Conclusion
We conclude that our antibiotic-eluting catheter may serve as a novel therapeutic option to treat empyema.
Keywords: pleural space infections, pleural drainage, drug-eluting catheter, nanofibers, penicillin, sustained release
Introduction
Empyema is a common medical problem, and more than 65,000 new cases are diagnosed in the UK and USA each year.1,2 Despite advances in medical management, mortality from empyema remains high (10% to 20%).3,4 Approximately 15% of patients fail to respond to standard treatments, ultimately requiring surgery.2,3
Inadequate intrapleural drug concentrations resulting from poor penetration of systemic antibiotics into the pleural cavity is a major cause of treatment failure in empyema. Although antibiotics are generally believed to be present in pleural fluid at levels that are comparable to those attained in serum after intravenous administration, clinically sound evidence remains limited. Most human studies supporting this conclusion were performed in patients with diseases other than empyema.5,6 Notably, the diffusion of antimicrobial agents in the pleural space in patients with empyema may be hindered by pleural thickening and a low pleural fluid pH. In this regard, rifampin concentrations in the pleural fluid have been reported to be less than 4% of those measured in the sera of patients with chronic tuberculous empyema.7 Similar results have been observed for aminoglycosides.5 Importantly, insufficient local concentrations of antimicrobial drugs could contribute to failure of antibiotic treatment and the development of antimicrobial resistance.8 To increase intrapleural antibiotic levels, repeated high-dose systemic administration is generally required. Unfortunately, such an approach can increase the likelihood of adverse drug reactions. In this scenario, intrapleural drug delivery may provide a useful means of achieving high therapeutic concentrations of antibiotics in the pleural space while maintaining a low systemic exposure.
To circumvent this issue, we have previously developed a novel local antibiotic drug delivery system based on biodegradable beads for the treatment of empyema.9 In vivo experiments demonstrated that this strategy ensured a fairly steady release of antimicrobial drugs in the pleural space for at least 2 weeks with minimal systemic toxicity. However, the clinical use of biodegradable beads may be limited by the requirement of a surgical implant procedure. Because most patients with pleural infections undergo drainage procedures, the local delivery of antimicrobial agents via a drainage tube may represent an ideal therapeutic strategy. Therefore, the aim of our study was to develop an antibiotic-eluting pigtail catheter coated with electrospun nanofibers for the sustained release of antibiotics in the pleural space. Electrospun nanofibers prepared using polylactide-polyglycolide (PLGA) copolymer and penicillin G sodium dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol were used to coat the surface of an Fr6 pigtail catheter. An elution method in combination with high-performance liquid chromatography (HPLC) was used to investigate the in vitro release pattern of penicillin from the catheter. In vivo studies were performed using rabbits treated either locally (Group 1, penicillin delivered through the antibiotic-eluting catheter) or systemically (Group 2, penicillin administered via intramuscular injection). Penicillin concentrations in serum and pleural fluid were then serially measured and compared. Histological examination of lung specimens was also performed.
Materials and methods
Penicillin-coated catheter fabrication
An Fr6 pigtail catheter was coated with electrospun penicillin-loaded nanofibers. To this aim, PLGA (50:50, Resomer RG 03, Boehringer Ingelheim, Ingelheim, Germany) and penicillin G sodium (Y F Chemical Corp., Taipei, Taiwan) in the amounts of 240 mg and 40 mg, respectively, were initially dissolved in 1 mL 1,1,1,3,3,3-hexafluoro-2-propanol. The solution was then delivered and electrospun through a syringe pump (volumetric flow rate, 3.6 mL/h) to coat the pigtail catheter with nanofibers. The laboratory setup of electrospinning for this study consisted of a syringe and needle (internal diameter, 0.42 mm), a ground electrode, a pigtail catheter mounted on a motor, a collection plate, and a high-voltage supply. The needle was connected to the high-voltage supply for generating positive DC voltages (up to 35 kV) and currents (up to 4.16 mA/125 W). The rotational speed of the motor was 300 rpm. The distance between the needle tip and the ground electrode was 10 cm, and the positive voltage applied to the polymer solution was 17 kV. All of the electrospinning experiments were performed at room temperature. After electrospinning, all nanofiber-mounted pigtail catheters were placed in a vacuum oven at 40°C for 72 hours to let the solvents evaporate. A penicillin G dose of 20 mg was used for each catheter. The amount of antibiotics coated on the catheter was determined as previously described.9
Scanning electron microscopy
The morphology of the electrospun nanofibers was analyzed using a Hitachi S3000N scanning electron microscope (SEM; Hitachi Ltd., Tokyo, Japan) after gold coating. Determination of the average nanofiber diameter and size distribution was performed from SEM images.
Standard curve of antibiotic concentrations
The antibiotic standard curve concentrations were determined using HPLC on a Waters 600 multisolvent delivery system (Waters Corporation, Milford, MA, USA). Penicillin was separated using a Phenomenex HPLC column (Waters). The mobile phase contained 0.01 M potassium dihydrogen phosphate, acetonitrile (Mallinckrodt Pharmaceuticals, Hazelwood, MO, USA), and methanol in a volume ratio of 72:18:10. The flow rate was 1.0 mL/min, and the absorbance was monitored at 225 nm. Penicillin solutions at five concentrations (0.1, 1, 10, 100, and 1,000 mg/mL) were analyzed by HPLC, and the peak areas were used to plot the standard curve.
In vitro penicillin release
The pattern of penicillin release from the antibiotic-loaded catheter was investigated in vitro using the elution method. Segments measuring 1 cm in length of the antibiotic/polymer-coated catheter were placed in glass test tubes containing 1 mL phosphate-buffered saline (0.15 mol/L, pH 7.4). The test tubes were incubated at 37°C for 24 hours and the eluent was then collected and analyzed. Fresh phosphate-buffered saline (1 mL) was added, and the tubes were incubated for another 24 hours. This procedure was repeated for a total of 30 days. The penicillin concentration in the eluent was calculated from the HPLC standard curve.
In vivo penicillin release
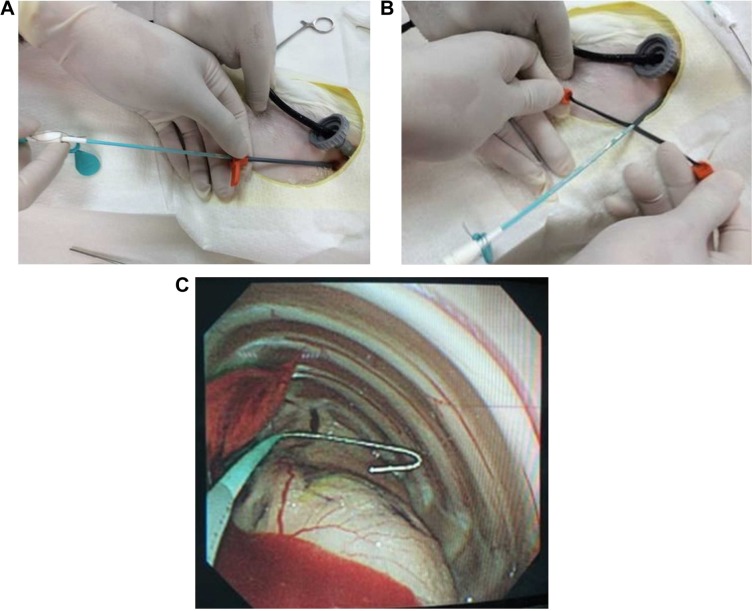
Eight healthy New Zealand White rabbits with an average weight of 2.5 kg were used for in vivo experiments. All animal procedures were reviewed and approved by the Institutional Animal Case and Use Committee of the Chang Gung University (IACUC approval number: CGU12-071). Rabbits were divided into two groups (n=4 each); Group 1 was treated with the penicillin-eluting catheter (20 mg/catheter), and Group 2 received systemic intramuscular administration of penicillin (10 mg/kg every 2 days with a non-drug eluting catheter). In brief, all rabbits were anesthetized using an intraperitoneal injection of ketamine (75 mg/kg) and acepromazine (2.5 mg/kg). Additional intraperitoneal injections were given to maintain or re-induce general anesthesia throughout the entire procedure. The rabbits breathed spontaneously and their body temperatures were maintained with blankets and heat lamps. A single 5 mm incision was made on the back of each rabbit to allow the insertion of a 5 mm Thoracoport (Covidien, Mansfield, MA, USA). A 3 mm flexible fiberoptic bronchoscope was placed into the pleural space through one port to confirm the presence of pneumothorax and to monitor the entire procedure. A 3 mm incision was subsequently made lateral to the first for guide wire insertion (Seldinger technique).10 The dilator and peel-away sheath were then inserted over the guide wire into the pleural space. Upon removal of the dilator, the catheter was promptly inserted through the peel-away sheath (Figure 1A). The sheath was peeled away (Figure 1B), and proper catheter placement was confirmed using fiberoptic bronchoscopy (Figure 1C). The incision was sutured with 2–0 Prolene, and the external end of the catheter was connected to a collecting bag, which was changed on a daily basis. Venous blood samples drawn from the rabbits’ ears were collected for analysis. Penicillin concentrations in both the pleural fluid (collected daily from day 0 to day 21) and venous blood (collected on days 0, 1, 2, 3, 7, 14, and 21) were determined using HPLC.
Figure 1.
Photographically, the deployment process of the drug-eluting catheter (A) Upon removal of the dilator, the catheter was promptly inserted through the peel-away sheath. (B) The sheath was peeled away. (C) Confirmation of the proper catheter placement by fiberoptic bronchoscopy.
Histopathology
On day 21, all rabbits were sacrificed. Lungs were removed, fixed in 20% formalin, sectioned into blocks, dehydrated in graded concentrations of ethanol, and finally embedded in paraffin. Serial sections were cut at 2 mm, stained with hematoxylin–eosin, and examined using light microscopy. An experienced pathologist blinded to the treatment protocol performed all morphometric examinations of the lungs. The presence and extent of lung injury were determined using a scoring system as previously described.11 The following four pathological processes were scored on a 5-point (0–4) scale: a) alveolar congestion, b) hemorrhage, c) leukocyte infiltration or aggregation of neutrophils in the airspace or vessel wall, and d) thickness of the alveolar wall. A score of 0 indicated a normal finding; 1, mild (<25%) lung involvement; 2, moderate (25%–50%) lung involvement; 3, severe (50%–75%) lung involvement; and 4, very severe (>75%) lung involvement. A global lung injury score was obtained by summing all these scores. Finally, a mean (± standard deviation) score was generated from all the analyzed lungs.
Statistical analysis
Penicillin concentrations in pleural fluid and serum of Group 1 and Group 2 rabbits were compared using a repeated measures analysis of variance (ANOVA) with the Greenhouse–Geisser correction (because of the small sample size). Lung injury scores were compared using the Student’s t-test. All calculations were performed using SPSS statistical software (SPSS Inc., Chicago, IL, USA). Two-tailed P-values <0.05 were considered statistically significant.
Results
Antibiotic-eluting pigtail catheters coated with electrospun nanofibers were successfully fabricated with the electrospinning technique (Figure 2A). Figure 2B shows the SEM images of the drug-eluting PLGA nanofibers (20,000× magnification). The electrospun nanofibers had high porosity and their diameters ranged from 80 to 630 nm.
Figure 2.
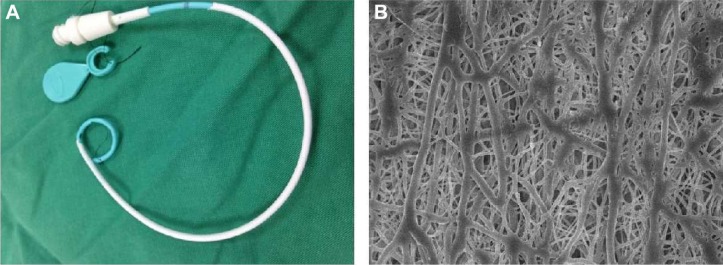
Appearance of the pigtail catheter.
Notes: (A) Gross appearance of the catheter. (B) Scanning electron microscopy findings of the drug-eluting PLGA nanofibers (20,000× magnification).
In vitro antibiotic release
Figure 3 shows the release curve of penicillin from the catheter according to in vitro experiments. There was a burst release of penicillin (10% of the total dose) in the first 24 hours, followed by a sustained release of a 60% dose in the subsequent 30 days. Antibiotic concentrations remained above the minimum inhibitory concentration (MIC) breakpoint for pneumococcal pneumonia (2 μg/mL) throughout the entire study period.12 Furthermore, penicillin levels were higher than the resistance breakpoint (8 μg/mL).12 These results suggest that the antibiotic-eluting pigtail catheter coated with electrospun nanofibers was capable of delivering a penicillin dose sufficient for eradicating both susceptible and resistant strains.
Figure 3.
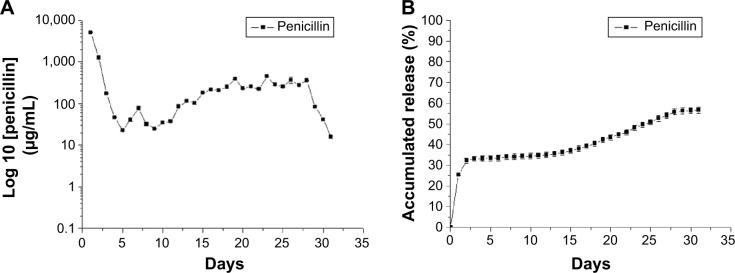
In vitro release curve of the penicillin-eluting catheter.
Notes: (A) Daily release curve. (B) Sustained release curve.
In vivo antibiotic release
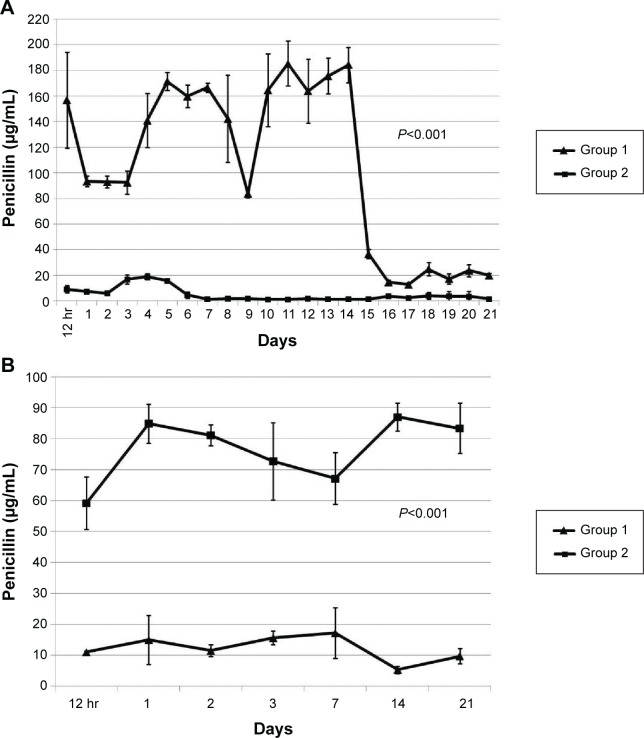
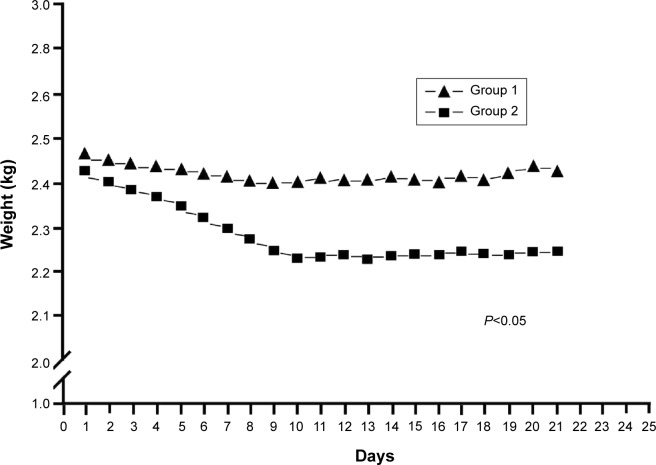
Figure 4 shows the patterns of in vivo release. Penicillin levels in the pleural fluid were significantly higher in Group 1 than in Group 2, with concentrations being markedly above the MIC in both cases (Figure 4A, P<0.001). Drug concentrations abruptly dropped after 14 days because of a tube obstruction by a fibrin clot. The severity of the systemic toxicity (as reflected by serum penicillin levels) was higher in Group 2 and barely detectable in Group 1 (Figure 4B, P<0.001). Consequently, Group 2 rabbits showed paralytic ileus. The systemic antibiotic dosage was reduced to 5 mg/kg on day 10 because of significant weight loss (Figure 5, P<0.05). No animals died during the study period. Figure 6A and B show the lung histological findings in Group 1 and Group 2, respectively. No significant intergroup difference was observed in terms of lung injury scores (P>0.05).
Figure 4.
In vivo release curve of penicillin (A) in the pleural fluid and (B) in the blood. Group 1 was treated with the penicillin-eluting catheter (20 mg/catheter), and Group 2 received systemic intramuscular administration of penicillin (10 mg/kg every 2 days with a non-drug eluting catheter).
Figure 5.
Body weight change in the experimental groups. Group 1 was treated with the penicillin-eluting catheter (20 mg/catheter), and Group 2 received systemic intramuscular administration of penicillin (10 mg/kg every 2 days with a non-drug eluting catheter).
Figure 6.
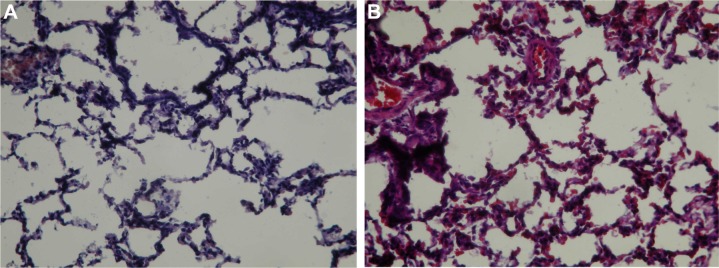
Pathological examination of lung tissues.
Notes: (A) Group 1 rabbits showed interstitial inflammatory cell infiltrates and pneumocyte hyperplasia (hematoxylin–eosin staining, 100× magnification). (B) Group 2 rabbits showed a pattern very similar to that observed in Group 1 (hematoxylin–eosin staining, 100× magnification). Group 1 was treated with the penicillin-eluting catheter (20 mg/catheter), and Group 2 received systemic intramuscular administration of penicillin (10 mg/kg every 2 days with a non-drug eluting catheter).
Discussion
It has been several years since the idea of using chest tubes for delivering antibiotics into the pleural space of patients with empyema was proposed.13–15 Unfortunately, the need for repeated injections is technically demanding, ultimately limiting its widespread adoption in routine practice. In this scenario, a drug-eluting tube that can allow both pleural drainage and the simultaneous delivery of therapeutic agents would represent an ideal alternative. Accordingly, the idea of using antibiotic-impregnated catheters is not novel and several antibiotic-eluting devices are currently commercially available.16,17 However, the primary goal of such products is to deliver low-dose antimicrobial drugs for preventing catheter-related infections rather than to treat regional infectious processes. We presented herewith the development of an antibiotic-eluting pigtail catheter coated with electrospun nanofibers capable of ensuring a steady delivery of bactericidal penicillin in the pleural cavity for at least 2 weeks with minimal systemic exposure. We believe that this novel drug delivery system may represent a potential treatment option for empyema.
Compared with conventional coating methods (eg, dip coating or spray coating), electrospinning is a remarkably simple method that allows effective coating with few, if any, limitations to the substrate materials.18,19 Electrospinning allows modifying the morphology of the electrospun nanofiber matrix as well as the structure surface, ultimately enabling a number of different modifications.20 Consequently, the described technique can be used for encapsulating various nanoparticles or nanofillers into a nanofiber matrix.21 Different types of molecules (including small interfering ribonucleic acid [siRNA] and proteins) can be easily coated onto the catheter surface by using sheath-core structured nanofibers, without significantly affecting the bioactivity of these drugs.22 During the electrospinning process, a polymeric solution placed inside a syringe was driven out from a metal capillary connected to a high-voltage power supply. After solvent evaporation, nanofibers were collected in the form of a non-woven matrix on the catheter. Continuous pharmaceutical nanofibers were then obtained when their concentrations were sufficient to generate significant chain entanglements in the polymers. Notably, the cellular uptake of nanoscale fibers (particularly with a size of ~100 nm) is 15 to 250 times faster than that of micrometer-sized fibers.23 The payload can be fine-tuned using different PLGA/penicillin molar ratios. Notably, drug loading had a significant impact on the drug release pattern. All of these features ensured a high and sustained penicillin release from the catheter (significantly above the MIC breakpoint both in vivo and in vitro). We have previously shown that the diameter distribution of drug-eluting nanofibers can be modulated through various processing parameters (eg, solvent, polymer concentration, ratio of drug loading, and flow rate). However, the role played by nanofiber diameters on drug release was limited.24
Compared to the systemic administration of antimicrobial drugs, highly localized administration of intrapleural antibiotics may be associated with a lower incidence of adverse systemic effects (as shown in our in vivo experiments, Figure 5) and a reduced likelihood of developing antimicrobial resistance. Our catheter for antibiotic delivery may also have additional advantages. First, its antimicrobial coating may be tailored for optimal efficacy based on culture and antibiogram of the pleural fluid. In this regard, the high degrees of biocompatibility, safety, and versatility of PLGA allow successful coating of many different water-soluble antibiotics. Second, electrospun nanofibers do not cause major conformational changes in the catheter, allowing its safe and reproducible image-guided insertion into the pleural cavity.
In general, the release kinetics of drugs from biodegradable devices comprises three phases consisting of an initial burst, a diffusion-controlled release, and a degradation-controlled phase. During the manufacturing process, most drugs are dispersed into the bulk of the PLGA matrix; however, some pharmaceutical compounds may be located on the nanofiber surface (ultimately causing the initial burst of drug release). An initial burst of drug release from antibiotic-eluting devices is desirable to mimic the high loading doses used in systemic antibiotic therapy. Notably, in vitro experiments confirmed such a release pattern for our antibiotic-eluting pigtail catheter (Figure 3). However, no obvious initial burst release was observed in animal experiments. This observation may be explained by the fact that metabolic rates for all pharmaceuticals are invariably lower in vivo than in vitro. In addition, binding of penicillin to proteins may result in different in vivo pharmacokinetics as compared to in vitro findings.
Some caveats of our study merit comment. First, the in vivo assessment of sustained penicillin release from the catheter into the pleural cavity was performed in healthy rabbits. The question as to whether pleural infections could have an impact on the observed pharmacokinetics remains open. Second, current animal models used for assessing the performance of drug-eluting catheters are limited in their ability to replicate human conditions. Specifically, future studies are needed to clarify whether our results obtained in the rabbit can be extrapolated to humans, because rabbits are a species with thin visceral pleura, whereas humans have thick visceral pleura.
Conclusion
In summary, we presented herewith an antibiotic-eluting pigtail catheter coated with electrospun nanofibers that ensure a steady delivery of penicillin in the pleural space for at least 2 weeks with minimal systemic exposure. We conclude that this novel drug delivery system may be an option for treatment of empyema.
Acknowledgments
We gratefully acknowledge the Chang Gung Memorial Hospital (Linkou, Taiwan) for financially supporting this research through contract numbers CMRPD290071 and CMRPG3E1091.
Footnotes
Disclosure
The authors declare no conflicts of interest.
References
- 1.Davies HE, Davies RJ, Davies CW, BTS Pleural Disease Guideline Group Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii41–ii53. doi: 10.1136/thx.2010.137000. [DOI] [PubMed] [Google Scholar]
- 2.Maskell NA, Batt S, Hedley EL, Davies CW, Gillespie SH, Davies RJ. The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Respir Crit Care Med. 2006;174(7):817–823. doi: 10.1164/rccm.200601-074OC. [DOI] [PubMed] [Google Scholar]
- 3.Farjah F, Symons RG, Krishnadasan B, Wood DE, Flum DR. Management of pleural space infections: a population-based analysis. J Thorac Cardiovasc Surg. 2007;133(2):346–351. doi: 10.1016/j.jtcvs.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Maskell NA, Davies CW, Nunn AJ, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med. 2005;352(9):865–874. doi: 10.1056/NEJMoa042473. [DOI] [PubMed] [Google Scholar]
- 5.Thys J, Serruys-Schoutens E, Rocmans P, Herchuelz A, Vanderlinden M, Yourassowsky E. Amikacin concentrations in uninfected postthoracotomy pleural fluid and in serum after intravenous and intrapleural injection. Chest. 1984;85(4):502–505. doi: 10.1378/chest.85.4.502. [DOI] [PubMed] [Google Scholar]
- 6.Hughes CE, Van Scoy RE. Antibiotic therapy of pleural empyema. Semin Respir Infect. 1991;6(2):94–102. [PubMed] [Google Scholar]
- 7.Elliott A, Beming S, Iseman M, Peloquin C. Failure of drug penetration and acquisition of drug resistance in chronic tuberculous empyema. Tuber Lung Dis. 1995;76(5):463–467. doi: 10.1016/0962-8479(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 8.Teixeira LR, Sasse SA, Villarino MA, Nguyen T, Mulligan ME, Light RW. Antibiotic levels in empyemic pleural fluid. Chest. 2000;117(6):1734–1739. doi: 10.1378/chest.117.6.1734. [DOI] [PubMed] [Google Scholar]
- 9.Liu KS, Liu SJ, Chen HY, et al. Steady antibiotic release from biodegradable beads in the pleural cavity: an in vitro and in vivo study. Chest. 2012;141(5):1197–1202. doi: 10.1378/chest.11-1254. [DOI] [PubMed] [Google Scholar]
- 10.Higgs Z, Macafee D, Braithwaite B, Maxwell-Armstrong C. The Seldinger technique: 50 years on. Lancet. 2005;366(9494):1407–1409. doi: 10.1016/S0140-6736(05)66878-X. [DOI] [PubMed] [Google Scholar]
- 11.Belperio JA, Keane MP, Burdick MD, et al. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest. 2002;110(11):1703–1716. doi: 10.1172/JCI15849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wikler MA. Performance standards for antimicrobial susceptibility testing: eighteenth informational supplement. Clinical and Laboratory Standards Institute (CLSI); 2008. [Google Scholar]
- 13.Mennander A, Laurikka J, Kuukasjärvi P, Tarkka M. Continuous pleural lavage may decrease postoperative morbidity in patients undergoing thoracotomy for stage 2 thoracic empyema. Eur J Cardiothorac Surg. 2005;27(1):32–34. doi: 10.1016/j.ejcts.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Ng T, Ryder BA, Maziak DE, Shamji FM. Treatment of postpneumonectomy empyema with debridement followed by continuous antibiotic irrigation. J Am Coll Surg. 2008;206(3):1178–1183. doi: 10.1016/j.jamcollsurg.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Karmy-Jones R, Sorenson V, Horst HM, Lewis JW, Jr, Rubinfeld I. Rigid thorascopic debridement and continuous pleural irrigation in the management of empyema. Chest. 1997;111(2):272–274. doi: 10.1378/chest.111.2.272. [DOI] [PubMed] [Google Scholar]
- 16.Hanna HA, Raad II, Hackett B, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030–1038. doi: 10.1378/chest.124.3.1030. [DOI] [PubMed] [Google Scholar]
- 17.Sciubba DM, Stuart RM, McGirt MJ, et al. Effect of antibiotic-impregnated shunt catheters in decreasing the incidence of shunt infection in the treatment of hydrocephalus. J Neurosurg. 2005;103(Suppl 2):131–136. doi: 10.3171/ped.2005.103.2.0131. [DOI] [PubMed] [Google Scholar]
- 18.Yu DG, White K, Chatterton N, Li Y, Li L, Wang X. Structural lipid nanoparticles self-assembled from electrospun core–shell polymeric nanocomposites. RSC Adv. 2015;5(13):9462–9466. [Google Scholar]
- 19.Liu SJ, Hsiao CY, Chen JK, Liu KS, Lee CH. In-vitro release of anti-proliferative paclitaxel from novel balloon-expandable polycaprolactone stents. Mater Sci Eng C Mater Biol Appl. 2011;31(5):1129–1135. [Google Scholar]
- 20.Yu DG, Zhou J, Chatterton NP, Li Y, Huang J, Wang X. Polyacrylonitrile nanofibers coated with silver nanoparticles using a modified coaxial electrospinning process. Int J Nanomedicine. 2012;7:5725–5732. doi: 10.2147/IJN.S37455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu DG, Zhu LM, White K, Branford-White C. Electrospun nanofiber-based drug delivery systems. Health. 2009;1(2):67–75. [Google Scholar]
- 22.Nguyen TT, Chung OH, Park JS. Coaxial electrospun poly(lactic acid)/chitosan (core/shell) composite nanofibers and their antibacterial activity. Carbohydr Polym. 2011;86(4):1799–1806. [Google Scholar]
- 23.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res. 2009;26(1):235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 24.Chen DW, Hsu YH, Liao JY, Liu SJ, Chen JK, Ueng SW. Sustainable release of vancomycin, gentamicin and lidocaine from novel electrospun sandwich-structured PLGA/collagen nanofibrous membranes. Int J Pharm. 2012;430(1–2):335–341. doi: 10.1016/j.ijpharm.2012.04.010. [DOI] [PubMed] [Google Scholar]