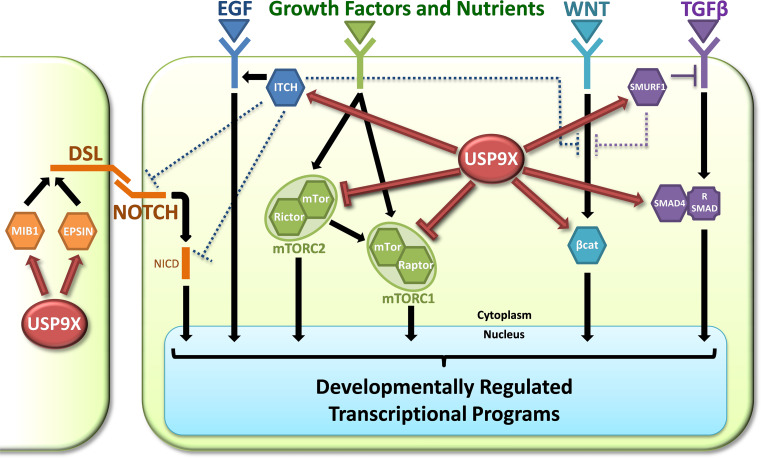
Fig. 2.
Coordination of developmental signalling pathways by USP9X. Interactors of USP9X (hexagons) are key regulators, or signal transduction molecules, of signalling pathways important for many aspects of embryogenesis. In Notch signalling (orange), USP9X is required in the signal sending cells to antagonise the proteasomal degradation of both Mind bomb1 (MIB1) and Epsin1, which control key endocytic events of Notch ligands (Delta, Serrate and Lag2; DSL) required for signalling competence. In EGF signalling (dark blue), USP9X protects ITCH from proteasomal degradation, which then inhibits the delivery of the EGF receptor to the lysosome and hence promotes signalling output. USP9X interacts with multiple components of the mTOR signalling pathway (green) including mTOR, Raptor and Rictor. Although the direct consequence of these interactions is unknown, Usp9x negatively regulates the signalling output of the two mTOR signalling complexes, mTORC1 and 2. USP9X can promote canonical Wnt signalling (light blue) by protecting the central signal transduction component β-catenin (βcat) from proteasomal degradation, thus promoting its accumulation, which is associated with nuclear translocation. USP9X can also affect TGFβ signalling (purple) both positively and negatively. USP9X protects SMURF1 from proteasomal degradation, which enables it to directly downregulate TGFβ receptors from the cell surface. In contrast, USP9X promotes TGFβ signalling by reversing the effects of mono-ubiquitylation of the common Smad4, which inhibits its ability to form signal transducing complexes with receptor SMADs (R SMAD). Adding further complexity, USP9X substrates can themselves regulate multiple signalling pathways, for example ITCH also negatively regulates Notch and Wnt signalling (dark blue dotted lines), whilst SMURF1 also negatively regulates Wnt signalling (purple dotted lines)