Abstract
Smoking is associated with higher infertility risk. The aim of this study was to evaluate protective effects of melatonin on the uterus and oviduct in mice exposed to nicotine. Adult female mice (n=32) were divided into four groups. Group A: control animals received normal saline, Group B: injected with nicotine 40µg/kg, Group C: injected with melatonin 10 µg, Group D: injected with nicotine 40µg/kg and melatonin 10 µg. All animals were treated over 15 days intraperitoneally. On the 16th day, animals in the estrus phase were dissected and their uterus and oviducts were removed. Immunohistochemistry was recruited for studying apoptosis and for detection of estrogen receptor (ER) alpha in luminal epithelium of the uterus and oviduct. Enzyme-linked immunosorbent assay was used for serum estradiol level determination. Nicotine in group B decreased estradiol level and ERalpha numbers both in the uterus and oviduct (p<0.05). Co-administration of melatonin-nicotine in Group D ameliorated the histology of the uterus and oviduct, increased ERalpha numbers and reduced apoptosis in the uterus and oviduct compared with the nicotine Group B (p<0.05). This study indicates that nicotine impairs the histology of the uterus and oviduct and co-administration of melatonin-nicotine ameliorates these findings, partly through alteration in ERalpha numbers and reduction of apoptosis.
Keywords: nicotine, melatonin, uterus, oviduct, estrogen receptor
Introduction
Cigarette smoking is associated with reproductive life impairment, such as earlier onset of menopause, higher infertility risk, lower fecundity rate and lower in vitro fertilization (IVF) success rate (Dechanet et al., 2011).
Nicotine is considered one of the most important components of cigarette smoke. Several studies have reported effects of nicotine on the endometrium and myometrium (Dechanet et al., 2011). Nicotine impairs fertility, reduces uterus weight, endometrial and myometrial thickness (Tuttle et al., 2009) with a direct effect on the morphology of ovaries and estradiol levels in rats (Sanders et al., 2002) or mice (Mohammadghasemi et al., 2012). Fetal or neonatal exposure to nicotine was found to induce apoptosis in rat ovary granulosa cells (Petrik et al., 2009). The morphology and functional integrity of the oviducts and uterus are evidently estrogen dependent. Estrogen plays key roles in the development and maintenance of normal sexual and reproductive functions (Heldring et al., 2007). The biological effects of 17β-estradiol (E2) are mediated through activation of estrogen receptors (ERs), a ligand-dependent transcription factor belonging to the nuclear hormone receptor super-family (Shao et al., 2012). In rodents and mammals, ERalpha is the predominant ER subtype in the fallopian tubes and uterus and plays a crucial role in physiological processes of the female reproductive tract (Wang et al., 2000; Shao et al., 2012). Thus alteration in expression of ERalpha may be associated with both function and morphology of the uterus and oviducts. Previous studies reported that both smoking (Sanders et al., 2002; Dechanet et al., 2011) and nicotine alone reduced the estradiol level (Petrik et al., 2009).
It is well known that melatonin, a pineal hormone, plays a key role in regulating several reproductive processes (Tamura et al., 2009). It is a powerful antioxidant and indirect free radical scavenger which can detoxify both reactive oxygen and reactive nitrogen species and stimulates antioxidant enzymes (Tamura et al., 2009). Melatonin receptors have been detected in the female reproductive tract including granulose cells, corpus luteum and myometrium (Tamura et al., 2009). Melatonin controls the growth of cells through modulation of estrogen and progesterone receptors and has a direct effect on the cell cycle, cell differentiation and gap junction (Abd-Allah et al., 2003; Garcia-Navarro et al., 2007). It regulates the expression of sex steroid receptors in female reproductive tissues (Chuffa et al., 2013). It inhibits apoptosis (Ghasemi et al., 2010) and affects steroidogenesis through melatonin MT1 and MT2 receptors in the female reproductive tract (Woo et al., 2001). Under treatment with nicotine, exogenous melatonin was found to exert a protective function on the ovary (Mohammadghasemi et al., 2012). Most of the previous studies evaluated the effect of cigarette smoking on female fertility, yet little is known about the effect of nicotine on the endometrium or oviduct.
The aim of this study was to investigate the protective effect of simultaneous administration of a low dose of 10µg melatonin on the uterus and oviduct in mice under chronic treatment with 40µg/kg nicotine, one of the most important ingredients of cigarettes.
Materials and methods
Animals and treatment
Female adult NMRI mice (35–45 g) were purchased from Razi Institute, Karaj-Iran. All animals were housed in groups of eight to ten in cages under standard lighting conditions and with free access to water and food at 25°C. The animals were maintained and handled according to the protocols approved by the Guilan University of Medical Sciences Animal Care and Use Committee. Initially, the animals were randomly divided into four groups. The first group: control animals treated with normal saline, the second group: animals were injected with nicotine 40µg/kg (Sigma, USA). The third group: animals were injected with melatonin (Sigma, USA), 10µg. The fourth group: animals were injected with nicotine 40µg/kg and melatonin 10µg. In all groups the animals were injected over 15 days. All injections were performed intraperitoneally.
Melatonin was dissolved in 95% ethanol and 0.9% NACL (1:7v/v) and was administered between 5.00–6.30 pm. After the melatonin and nicotine treatment period, all animals were monitored by vaginal smears. The estrous cycle was characterized by four phases: proestrus with numerous nucleated epithelial cells, some squamous epithelial cells and few leukocytes, estrus with many clusters of squamous epithelial cells, metestrus with some nucleated and squamous epithelial cells and abundant leukocytes, and diestrous with few cells and presence of thick mucus.
Animals in the estrus phase were chosen and placed in 4 groups, each comprising 8 mice. On day 16, all animals were dissected and their blood samples were collected. Their uterus (part nearer to the ovary) and oviducts were removed. Tissue samples were fixed in 10% neutral buffered formalin and were processed routinely for histological studies. Then 5-µm sections were prepared and stained with H&E or for immunohistochemical assessments to detect apoptosis and ERalpha luminal epithelial cells.
Histologic study
In each animal 2–3 slides and in each slide 5 microscopic fields were observed using a light microscope (Olympus Japan) with a magnification of 400×. The numbers of endometrial glands in the uterus or mucosal folds in the oviduct were counted.
Hormone measurement
Blood samples were collected through the inferior vena cava immediately after sacrificing the mice. The serum was separated and stored at –80°C. Estradiol levels were measured using an enzyme linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (Monobind,USA). Serum references (25µL) were dispensed into the wells. After adding the estradiol biotin reagent (50µL) into the wells, microplates were swirled, covered and incubated for 30 minutes at room temperature. In the next step, estradiol enzyme reagent (50µL) was added to each well and swirled, covered and incubated for 90 minutes at room temperature. After discarding the contents of the microplates, 350µL wash buffer was added and this was repeated three times. A dose of 100µL substrate solution was added to the wells and incubated at room temperature for 20 minutes. Finally, stop solution (50µL) was added to each well for stopping the enzymatic reaction and then the absorbance of each well was read at 450 nm.
Detection of ERalpha
Sections were dewaxed and rehydrated. Deparaffinization was carried out in xylene and graded ethanols. Endogenous peroxidase was blocked by incubation in 5% H2O2 in methanol for 15 min. Heat-mediated antigen retrieval was performed by microwaving three times for 5 min at 500W in Citra-Plus solution (Biogenex; San Ramon, Ca) for ER staining. Unspecific bindings were blocked using the biotin blocking system (Dako; Carpinteria,Ca). Incubation was carried out with the mouse monoclonal antibody1D5, dilution 1:200 (Dako), 1h at room temperature. Sections were then counterstained with hematoxylin, dehydrated, and observed under a light microscope. Cells with a brown nucleus revealed the presence of ERalpha. Cells with a blue nucleus were considered negative for the presence of ERalpha. In each animal, 2 slides and in each slide 8–10 fields with an area of 1×1 mm2 were studied. In each field the number of ERalpha positive cells were counted and then divided by numbers of both positive and negative cells and expressed in percentages.
Detection of apoptotic cells
In situ detection of cells with DNA strand breaks was performed in formalin-fixed, paraffin-embedded tissue sections by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) according to the manufacturer's instructions (Roche, Germany), as described previously (Ghasemi et al., 2010). Cells with a dark-brown nucleolus were considered apoptotic. In each animal 2 slides and in each slide 8–10 fields with an area of 1×1mm2 were studied. In each field the number of positive apoptotic cells was counted and then divided by numbers of both positive and negative cells and expressed in percentages.
Statistical analysis
Data analyses were performed using SPSS version 13.0 for windows microsoft year 2010. The normality distribution of samples was tested using the Kolmogorov-Smirnov test. Then the data were analyzed by analysis of variance (ANOVA) and Tukey post-hoc tests. The value p<0.05 was considered evidence for statistical significance.
Results
Histological assessment
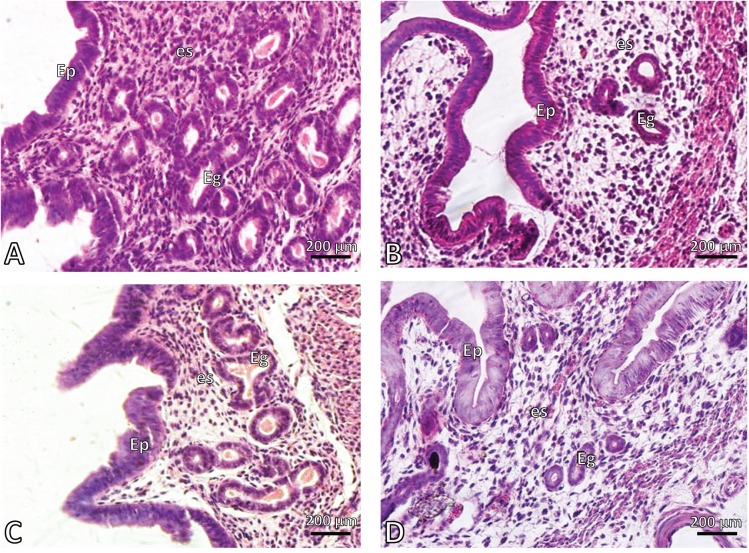
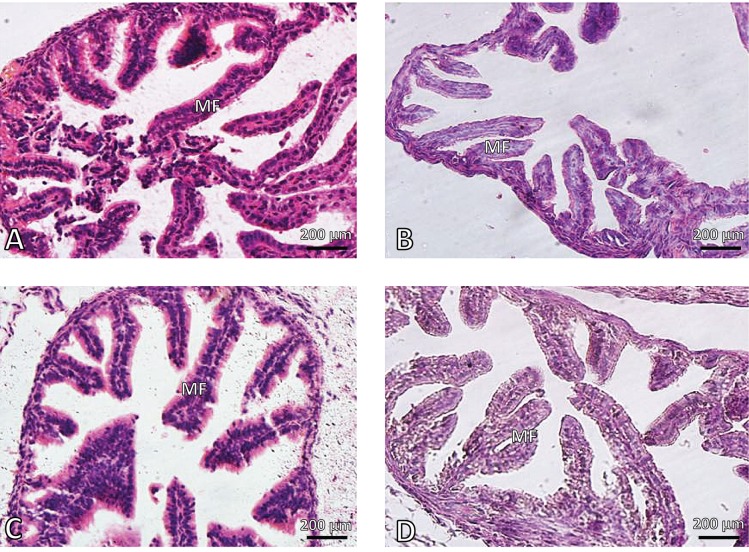
Nicotine induced morphological alterations in both the uterus endometrium and oviduct mucosa. The mean number of endometrial glands in the nicotine treated group was 6.4±6.2 vs 14.2±4.1 in the controls (p<0.05). Nicotine reduced significantly both endometrial glands and mucosal folds of the oviducts compared with the controls (p<0.05). Co-administration of nicotine-melatonin significantly increased the number of endometrial glands compared with the nicotine group (p<0.05) (Figures 1 – 2 and Table 1). The number of mucosal folds in the oviducts of the co-administered group was increased compared with the nicotine group, yet this value was not significant (Table 1).
Figure 1.
Photomicrograph of mouse endometrium. A: control, B: nicotine, C: melatonin and D: melatonin+nicotine treated mice. EP: epithelium, es: estroma, Eg: endometrial glands. H&E. Magnification 400×.
Figure 2.
Photomicrograph of mouse oviduct. A: control, B: nicotine, C: melatonin and D: melatonin+nicotine treated mice. Mf: mucosal folds. H&E. Magnification 400×.
Table 1.
Effect of nicotine and melatonin on adult mouse uterus and oviduct.
| O. folds Numbers/field | E. glands Numbers/field | Eralpha oviduct/field | ERalpha uterus/field | Estradiol level (pg/ml) | Groups |
|---|---|---|---|---|---|
| 18.2±3.4 | 14.2±4.1 | 11.00±2.20b | 15.25±1.46b | 57.25±4.43b | Control |
| 10.8±2.4a | 6.4±6.2ab | 4.25±1.03a | 10.56±1.63a | 41.37±3.70a | Nicotine |
| 16.3±3.1 | 12.1±3.0b | 13.00±2.44b | 12.25±1.05b | 53.25±5.14b | Melatonin |
| 15.2±4.0 | 10.6±2.8b | 7.66±2.53ab | 13.85±0.64b | 50.62±3.96ab | Nicotine+melatonin |
Data are expressed as Mean±S.D.
significant from controls p<0.05
significant from nicotine group p<0.05; E: Endometrial; O: Oviduct
Serum estradiol (E2) level and detection of ERalpha
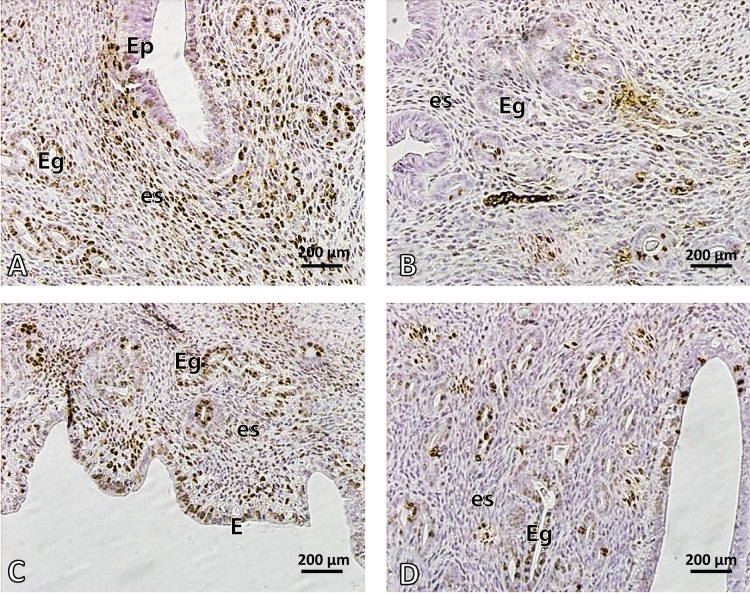
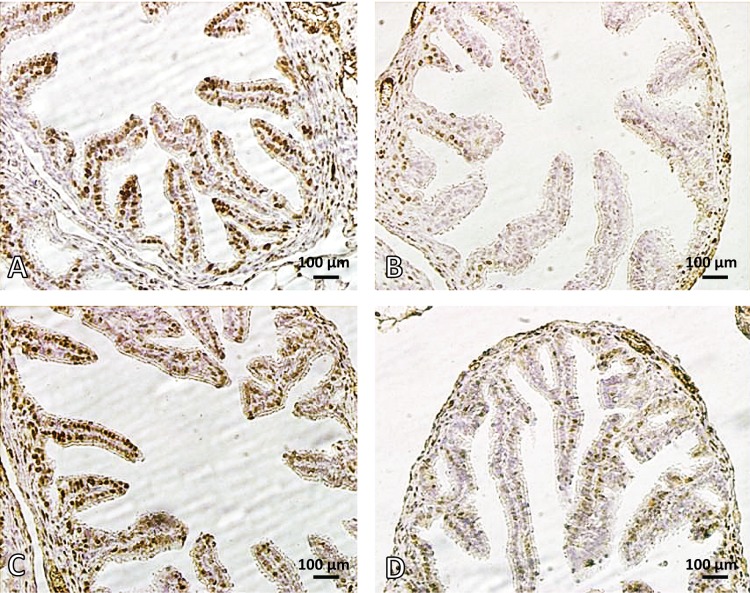
Administration of nicotine in the dose of 40µg/kg for 15 days reduced significantly the serum estradiol (E2) level and ERalpha in epithelial cells of the uterus and oviducts compared with the controls (p<0.05). Melatonin alone did not affect these parameters. However, co-administration of melatonin and nicotine increased significantly the serum estradiol (E2) level and ER alpha in epithelial cells of the uterus and oviducts compared with the nicotine group (p<0.05) (Table 1, Figures 3 – 4).
Figure 3.
Photomicrograph of mouse endometrium. A: control, B: nicotine, C: melatonin and D: melatonin+nicotine treated mice. Brown cells show ER alpha positive cells. Arrows show endometrial glands. Note the reduced numbers of ER alpha positive cells and endometrial glands in nicotine (B) compared with control (A). In D, melatonin ameliorated the endometrium and increased ERα. EP: epithelium, es: estroma, Eg: endometrial glands. Magnification 400×.
Figure 4.
Photomicrograph of mouse ampulla (oviduct). A: control, B: nicotine treated mouse, C: melatonin treated mouse and D: melatonin+nicotine treated mouse. Brown cells show ER alpha positive cells. Note the reduced numbers of ER alpha positive cells and mucosal folds (MF) in nicotine (B) compared with control (A). In D, melatonin ameliorated the mucosal folds of the oviduct and increased ER alpha. ER alpha Immunostaining. Magnification 400×.
Detection of apoptosis
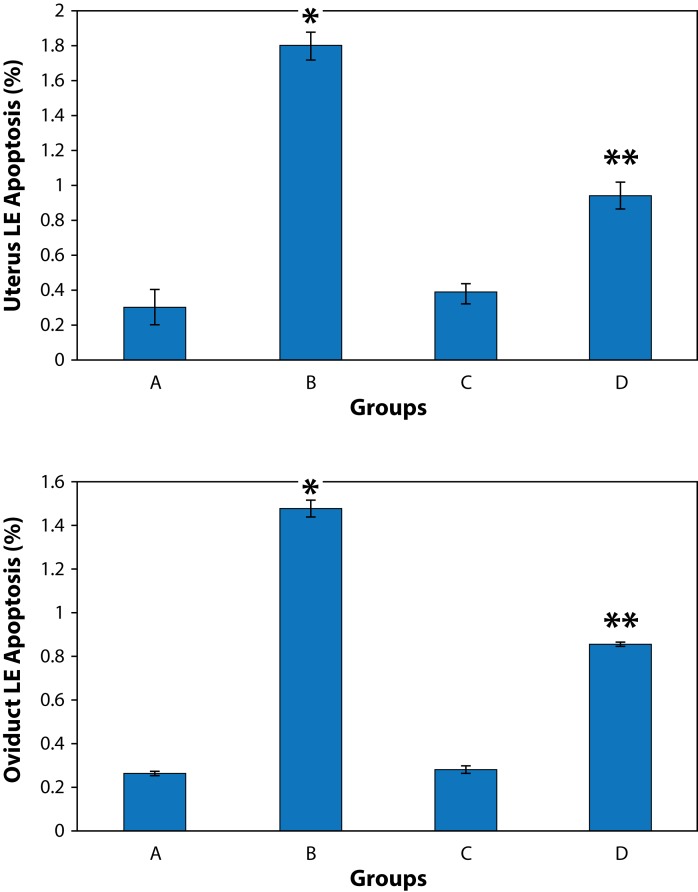
A few apoptotic cells were scattered in the luminal or glandular epithelium of the oviducts and uterus in controls. The percentage of apoptotic cells was significantly increased both in the uterus and oviducts in the nicotine treated group compared to controls (p<0.05). The nicotine-melatonin combination significantly reduced apoptosis in epithelial cells of the uterus and oviducts compared with the nicotine alone group (p<0.05) (Figure 5).
Figure 5.
Effect of nicotine and melatonin on apoptosis in luminal epithelium (LE) in mouse uterus and oviduct. A: control, B: nicotine, C: melatonin, D: melatonin+nicotine treated groups. Data are expressed as Mean± SD. * shows significant data from controls (A), p<0.05. ** shows significant data from both controls (A) and nicotine alone treated group (B), p<0.05.
Discussion
In this study, melatonin was found to exert protective effects on the uterus and oviduct in mice under treatment with nicotine. Nicotine administration was shown to be associated with a reduction in estradiol level and ERalpha numbers and it increased apoptosis both in the uterus and oviduct in mice.
Apoptosis is programmed physiological cell death that occurs in all types of cells in both embryonic and adult periods (Petrik et al., 2009). Due to the cyclic nature of the female reproductive system, the ovary, the endometrium and the mammary gland sustain continuous cycles of cell growth and apoptosis in response to hormonal changes (Meresman, 2011). Apoptotic mechanisms exploited by nicotine include alteration of the bcl2/bax ratio and activation of the caspase-3 pathway (Petrik et al., 2009). In this study, nicotine was found to induce an increase in the number of apoptotic cells in the epithelium of the uterus and oviduct. The increased level of apoptosis in the luminal epithelium of the uterus and oviduct is presumably due to a reduced level of estradiol and its receptor, as reported previously (Petrik et al., 2009; Wang et al., 2000). Our study showed that co-administration of melatonin-nicotine reduced apoptotic cells in luminal epithelial cells in the uterus and oviducts. Similarly, an antiapoptotic effect of melatonin was demonstrated in different tissues (Guneli et al., 2008; Take et al., 2009). The mechanisms related to antiapoptotic effects of melatonin include: bcl2 expression by mitochondrial pathways and reduction of caspase-3 activity (Tamura et al., 2009), antioxidative effect of melatonin, alteration in cell differentiation, growth factors, cell division and cell attachments (Garcia-Navarro et al., 2007), which were however not evaluated in the present study. A further study with focus on molecular mechanisms of apoptosis and interaction between melatonin receptors, estrogen receptors and nicotine acetylcholine receptors following treatment with nicotine and melatonin is therefore suggested.
Our study showed that nicotine reduced estradiol levels. Estrogen is primarily produced by preovulatory follicles under the influence of FSH in the rodent ovary (Byers et al., 1997; Chuffa et al., 2013). Estrogen has various intra-ovarian roles during follicogenesis. In granulosa cells of maturing follicles, estradiol has little effect alone but is required for maximum FSH stimulation of estradiol synthesis, LH receptor expression, LH responsiveness, antrum formation, gap junction formation, and prevention of atresia (Emmen et al., 2005). Smokers show abnormal endocrine profiles of testosterone, FSH, LH and estradiol levels (Dechanet et al., 2011). Epidemiologic studies reported that women who smoke had lower serum estrogen levels than nonsmokers (Barbieri et al., 2005). Increased estrogen hepatic metabolism and decreased estrogen synthesis are the probable mechanisms for the antiestrogenic effect of cigarette smoking (Michnovicz et al., 1986). In this study, the decreased estrogen level in the nicotine group can probably be explained by its toxic effects on the ovary or granulosa cell function (Mohammadghasemi et al., 2012). However, Sanders et al. (2002) did not find any changes in estradiol levels of the bovine theca interna and granulose cells following nicotine administration. In contrast, Gocze et al. (1997) and Bodies et al. (1997) observed a slight increase in estradiol production by human granulose cells treated with nicotine. One reason for these conflicting results may be due to species differences. Further reasons could include the source of cells and the length of culture period, variation in the form of the nicotine preparations used, as well as nicotine dosage and duration of nicotine exposure (Sanders et al., 2002).
In our study, ERalpha was reduced in the epithelial tissue of both the uterus and oviduct in nicotine treated animals. Steroid hormone action is mediated via nuclear receptor proteins ERalpha and ERbeta, which function as ligand modulated transcription factors. In mammals and rodents, the activity of ERalpha is regulated by cycling hormone levels (Wang et al., 2000). E2 was found to induce increased concentrations of both ER alpha and progesterone receptor (Wang et al., 2000). The lowered level of estradiol in this study reduced presumably the numbers of ERalpha. E2 was shown to enhance ER alpha mRNA synthesis in the rat uterus 24 h after injection and the uterine responses to E2 and progesterone are directly or indirectly mediated by the cell specific expression of their receptors (Wang et al., 1999).
Our study showed that co-administration of melatonin-nicotine increased both the estradiol level and ER alpha, compared with the group that received nicotine alone. Melatonin has specific receptors MT1 and MT2 both in the uterus and oviduct (Tamura et al., 2009). Depending on the tissue, organ and species, melatonin activates different second messenger cascades by interacting with the same receptor subtype (Tamura et al., 2009). Such alteration in the co-administered group may be due either to the direct effect of melatonin on both uterine and oviduct tissue or to the effect of melatonin on the hypothalamo-pituitary-gonadal axis (HPG). Melatonin interacts with estrogen-signaling pathways through indirect neuroendocrine mechanisms, direct actions at the tumor cell level, and regulation of enzymes involved in the biosynthesis of estrogens (Yoo & Jeung, 2009). It influences sex steroid production at different stages of ovarian follicular maturation (Adriaens et al., 2006). There are controversial results about the effect of melatonin on sex hormones. Melatonin treatment increases mRNA expression of LH but not FSH receptors in human granulose cells (Tamura et al., 2009). Melatonin increases the production of progesterone and androgen in porcine antral follicles, without effect on the estradiol level (Tanavde & Maitra 2003). However in hamsters, it increases ER activity in the uterus (Danforth et al., 1983) and decreases the production of progesterone and estradiol (Tamura et al., 2009). Abd-Allah et al. (2003) and Chuffa et al. (2013) found reduced numbers of ER in the rat uterus after administration of melatonin. Similarly, melatonin increasesd calbindin families of calcium-binding proteins expression through ERalpha. These conflicts are due to the cell type, dosage and duration of melatonin treatment, experimental model, and species (Tamura et al., 2009).
Our study showed co-administration of nicotine-melatonin reduced apoptosis and increased ER alpha compared to nicotine alone. These alterations probably suggest a functional link between increased cell survival and the up-regulation of ERalpha by melatonin induced ERalpha during nicotine-mediated cell death. In this regard, it has been shown that melatonin increases survival of rat pituitary GH3 cells against cell death mediated by H2O2 through ERalpha (Yoo & Jeung, 2009). Estrogen receptor alpha in other tissues also protects podocytes against apoptosis in vitro or in vivo (Kummer et al., 2011).
In conclusion, this study indicates that melatonin exerts a favorable effect on the uterus and oviduct in mice treated with nicotine through reduction of apoptosis and modification in ERalpha in the luminal epithelium of the uterus and oviduct. Our results also suggest that melatonin may have a significant beneficial effect for clinical applications and subfertility or infertility induced by smoking or nicotine replacement therapy in women. Future studies on the current topic in humans are therefore recommended.
REFERENCES
- Abd-Allah AR, El-Sayed el SM, Abdel-Wahab MH, Hamada FM. Effect of melatonin on estrogen and progesterone receptors in relation to uterine contraction in rats. Pharmacol Res. 2003;47:349–354. doi: 10.1016/s1043-6618(03)00014-8. [DOI] [PubMed] [Google Scholar]
- Adriaens I, Jacquet P, Cortvrindt R, Janssen K, Smitz J. Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. Toxicology. 2006;228:333–343. doi: 10.1016/j.tox.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Barbieri RL, Sluss PM, Powers RD, McShane PM, Vitonis A, Ginsburg E, Cramer DC. Association of body mass index, age, and cigarette smoking with serum testosterone levels in cycling women undergoing in vitro fertilization. Fertil Steril. 2005;83:302–308. doi: 10.1016/j.fertnstert.2004.07.956. [DOI] [PubMed] [Google Scholar]
- Bodis J, Hanf V, Torok A, Tinneberg HR, Borsay P, Szabo I. Influence of nicotine on progesterone and estradiol production of cultured human granulosa cells. Early Pregnancy. 1997;3:34–37. [PubMed] [Google Scholar]
- Byers M, Kuiper GG, Gustafsson JA, Park-Sarge OK. Estrogen receptor-beta mRNA expression in rat ovary: down-regulation by gonadotropins. Mol Endocrinol. 1997;11:172–182. doi: 10.1210/mend.11.2.9887. [DOI] [PubMed] [Google Scholar]
- Chuffa LG, Seiva FR, Favaro WJ, Amorim JP, Teixeira GR, Mendes LO, Fioruci-Fontanelli BA, Pinheiro PF, Martinez M, Martinez FE. Melatonin and ethanol intake exert opposite effects on circulating estradiol and progesterone and differentially regulate sex steroid receptors in the ovaries, oviducts, and uteri of adult rats. Reprod Toxicol. 2013;39:40–49. doi: 10.1016/j.reprotox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Danforth DN, Jr, Tamarkin L, Do R, Lippman ME. Melatonin-induced increase in cytoplasmic estrogen receptor activity in hamster uteri. Endocrinology. 1983;113:81–85. doi: 10.1210/endo-113-1-81. [DOI] [PubMed] [Google Scholar]
- Dechanet C, Anahory T, Mathieu Daude JC, Quantin X, Reyftmann L, Hamamah S, Hedon B, Dechaud H. Effects of cigarette smoking on reproduction. Hum Reprod Update. 2011;17:76–95. doi: 10.1093/humupd/dmq033. [DOI] [PubMed] [Google Scholar]
- Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER){alpha} and ER{beta} null mice indicate a role for ER{beta} in follicular maturation. Endocrinology. 2005;146:2817–2826. doi: 10.1210/en.2004-1108. [DOI] [PubMed] [Google Scholar]
- Garcia-Navarro A, Gonzalez-Puga C, Escames G, Lopez LC, Lopez A, Lopez-Cantarero M, Camacho E, Espinosa A, Gallo MA, Acuna-Castroviejo D. Cellular mechanisms involved in the melatonin inhibition of HT-29 human colon cancer cell proliferation in culture. J Pineal Res. 2007;43:195–205. doi: 10.1111/j.1600-079X.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- Ghasemi F, Faghani M, Khajehjahromi S, Bahadori M, Nasiri E, Hemadi M. Effect of Melatonin on proliferative activity and apoptosis in spermatogenic cells in mouse under chemotherapy. Journal of Reproduction & contraception. 2010;21:79–94. [Google Scholar]
- Gocze PM, Szabo I, Freeman DA. Influence of nicotine, cotinine, anabasine and cigarette smoke extract on human granulosa cell progesterone and estradiol synthesis. Gynecol Endocrinol. 1999;13:266–272. doi: 10.3109/09513599909167565. [DOI] [PubMed] [Google Scholar]
- Guneli E, Tugyan K, Ozturk H, Gumustekin M, Cilaker S, Uysal N. Effect of melatonin on testicular damage in streptozotocin-induced diabetes rats. Eur Surg Res. 2008;40:354–360. doi: 10.1159/000118032. [DOI] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Kummer S, Jeruschke S, Wegerich LV, Peters A, Lehmann P, Seibt A, Mueller F, Koleganova N, Halbenz E, Schmitt CP, Bettendorf M, Mayatepek E, Gross-Weissmann ML, Oh J. Estrogen receptor alpha expression in podocytes mediates protection against apoptosis in-vitro and in-vivo. PLoS One. 2011;6:e27457. doi: 10.1371/journal.pone.0027457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meresman G. Relevance of apoptosis in the female reproductive system. Invest Clin. 2011;52:274–290. [PubMed] [Google Scholar]
- Michnovicz JJ, Hershcopf RJ, Naganuma H, Bradlow HL, Fishman J. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med. 1986;315:1305–1309. doi: 10.1056/NEJM198611203152101. [DOI] [PubMed] [Google Scholar]
- Mohammadghasemi F, Jahromi SK, Hajizadeh H, Homafar MA, Saadat N. The protective effects of exogenous melatonin on nicotine-induced changes in mouse ovarian follicles. J Reprod Infertil. 2012;13:143–150. [PMC free article] [PubMed] [Google Scholar]
- Petrik JJ, Gerstein HC, Cesta CE, Kellenberger LD, Alfaidy N, Holloway AC. Effects of rosiglitazone on ovarian function and fertility in animals with reduced fertility following fetal and neonatal exposure to nicotine. Endocrine. 2009;36:281–290. doi: 10.1007/s12020-009-9229-4. [DOI] [PubMed] [Google Scholar]
- Sanders SR, Cuneo SP, Turzillo AM. Effects of nicotine and cotinine on bovine theca interna and granulosa cells. Reprod Toxicol. 2002;16:795–800. doi: 10.1016/s0890-6238(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Shao R, Feng Y, Zou S, Weijdegard B, Wu G, Brannstrom M, Billig H. The role of estrogen in the pathophysiology of tubal ectopic pregnancy. Am J Transl Res. 2012;4:269–278. [PMC free article] [PubMed] [Google Scholar]
- Take G, Erdogan D, Helvacioglu F, Goktas G, Ozbey G, Uluoglu C, Yucel B, Guney Y, Hicsonmez A, Ozkan S. Effect of melatonin and time of administration on irradiation-induced damage to rat testes. Braz J Med Biol Res. 2009;42:621–628. doi: 10.1590/s0100-879x2009000700006. [DOI] [PubMed] [Google Scholar]
- Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan DX, Sugino N, Reiter RJ. Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril. 2009;92:328–343. doi: 10.1016/j.fertnstert.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Tanavde VS, Maitra A. In vitro modulation of steroidogenesis and gene expression by melatonin: a study with porcine antral follicles. Endocr Res. 2003;29:399–410. doi: 10.1081/erc-120026946. [DOI] [PubMed] [Google Scholar]
- Tuttle AM, Stampfli M, Foster WG. Cigarette smoke causes follicle loss in mice ovaries at concentrations representative of human exposure. Hum Reprod. 2009;24:1452–1459. doi: 10.1093/humrep/dep023. [DOI] [PubMed] [Google Scholar]
- Wang H, Eriksson H, Sahlin L. Estrogen receptors alpha and beta in the female reproductive tract of the rat during the estrous cycle. Biol Reprod. 2000;63:1331–1340. doi: 10.1095/biolreprod63.5.1331. [DOI] [PubMed] [Google Scholar]
- Wang H, Masironi B, Eriksson H, Sahlin L. A comparative study of estrogen receptors alpha and beta in the rat uterus. Biol Reprod. 1999;61:955–964. doi: 10.1095/biolreprod61.4.955. [DOI] [PubMed] [Google Scholar]
- Woo MM, Tai CJ, Kang SK, Nathwani PS, Pang SF, Leung PC. Direct action of melatonin in human granulosa-luteal cells. J Clin Endocrinol Metab. 2001;86:4789–4797. doi: 10.1210/jcem.86.10.7912. [DOI] [PubMed] [Google Scholar]
- Yoo YM, Jeung EB. Melatonin-induced estrogen receptor alpha-mediated calbindin-D9k expression plays a role in H2O2-mediated cell death in rat pituitary GH3 cells. J Pineal Res. 2009;47:301–307. doi: 10.1111/j.1600-079X.2009.00714.x. [DOI] [PubMed] [Google Scholar]