Figure 1.
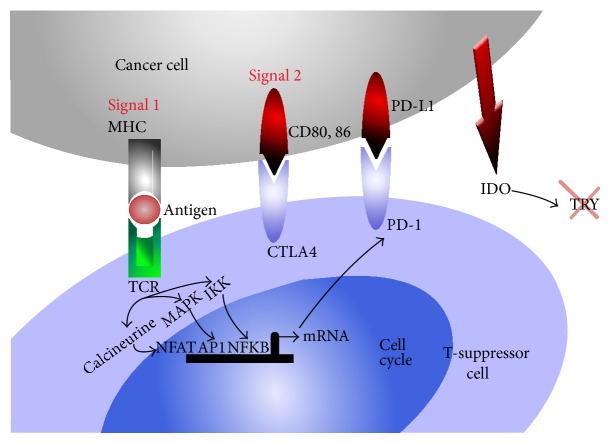
Cancer cell mediated immune suppression: upon ligation CTLA4 or PD-1 on suppressor cells, cancer cells produce 2,3 indolamine dioxygenase (IDO) and others (arginase, nitric oxide synthase) degrading amino acids arginine and tryptophan necessary for immune detection and elimination function of the effector CD8+ T-cells. Cancer cells undergo phenotypic or genomic modification under immune attack resulting in the survival and selection of variants that are capable of escaping immune attack. These modifications include HLA class 1, loss of tumor antigens, lack of death receptor signaling, regulatory T-cells, inhibitory cytokines, and immune check point molecules. Ligation of PD-L1 on the tumor cell surface results in tumor protection from cell death [82]. Interactions between PD-L1 and PD-1 in the tumor microenvironment protect the tumor through several distinct pathways including the ligation of PD-1 by PD-L1 on antigen specific T-cells leading to functional anergy and/or apoptosis of these effector T-cells [19, 83]. Effector T cells are further inhibited by PD-L1:CD80 interactions [18]. While PD-L1 interactions with PD-1 on cytotoxic CD8 T-cells dampen tumor specific effector immunity, PD-L1 PD1 interaction on T-regulatory cells (CD4+, CD25+) increases their suppressive function [35–37]. IDO: indolamine 2,3 deoxygenase; TCR: T cell receptor; MHC: major histocompatibility antigen; IL-2: interleukin-2; CTLA4 cytotoxic T lymphocytes antigen-4; PD-1: programmed death-1 also known as CD279; PD-L1 programmed death-ligand 1 known as B7-H1 or CD274; PD-L2 programmed death-ligand 2, known as CD273 or B7-DC B-7 dendritic cell; IFN-γ: interferon gamma; IL-2: interleukin-2; APC: antigen presenting cells; TRY: tryptophan, AP-1: activator protein-1; NFAT: nuclear factor of activated T-cells; NFKB: nuclear factor kappa B; MAPK: mitogen activated protein kinase; IKK: I kappa B kinase; PI3K: phosphatidylinositol-4,5-biphosphate 3-kinase; CDK: cyclin dependant kinase; JAK3: Janus kinase 3; mTOR: mammalian target of rapamycin.