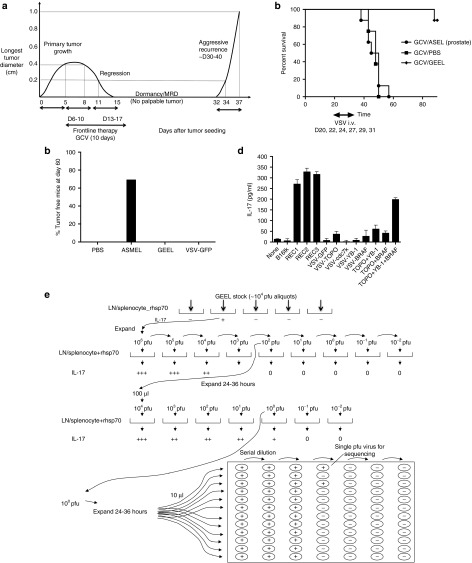
Figure 1.
VSV-expressed antigens treat recurrence. (a) Kinetics of the in vivo model of tumor treatment, regression, minimal residual disease, and recurrence. Representative data are shown from a C57BL/6 mouse seeded with a B16tk tumor at day 0. By day 5, tumor was established (longest tumor diameter 0.4 cm). GCV was then given i.p. at 50 mg/kg for 5 consecutive days (days 6–10), followed by 2 days rest, during which time tumor regressed (0.8 cm, day 8 and 0.2 cm at day 11). Five further consecutive injections of GCV were then given on days 13–17. With this regimen, most tumors regressed macroscopically by about days 15–20 after tumor seeding (day 15 undetectable in this mouse). Tumor regression was followed by later local recurrence in ~50–80% of the mice at different time points (typically between days 30–40) after the last injection of GCV. Once recurrence was palpable, the secondary tumor always grew very rapidly and much more aggressively than the primary tumor (from palpable (day 32) to 1.0 cm (day 37) in 5 days in this example). (b) C57BL/6 mice seeded with B16tk tumors 5 days previously were treated with GCV i.p. at 50 mg/kg for 5 consecutive days, followed by 2 days rest, followed by five further consecutive injections of GCV (days 6–10 and 13–17) as in a. Mice were injected i.v. with PBS or VSV-cDNA libraries (GEEL or ASEL, 107 pfu/injection) starting on day 20, by which time primary tumors had regressed (as in a), Subsequent i.v. injections were given on days 22, 24, 27, 29, and 31. Survival of mice (n = 7/8 per group) treated sequentially with GCV, then different combinations of VSV-cDNA as shown. Representative of two separate experiments. (c) C57BL/6 mice seeded with B16tk tumors 5 days previously were treated i.v. with PBS, VSV-cDNA libraries (ASMEL or GEEL), or with VSV-GFP (107 pfu/injection) on days 6, 8, and 10; 13, 15, and 17; 20, 22, and 24. Cumulative number of mice surviving tumor free at day 60 in two separate experiments is shown. (d) Splenocyte/LN cultures from tumor cured, GEEL-vaccinated mice were screened for IL-17 secretion following no infection/restimulation; following restimulation with freeze thaw cell lysates of B16tk, B16tk REC1, B16tk REC2, and B16tk REC3 cells; or following restimulation by infection (multiplicity of infection 10) with VSV-GFP; VSV-TOPO-IIα; VSV-cdc7k; VSV-YB-1; or VSV-BRAF; with a combination of VSV-TOPO-IIα + VSV-YB-1; or VSV-TOPO-IIα + VSV-BRAF; or with a combination of VSV-TOPO-IIα + VSV-YB-1 + VSV-BRAF. (e) Functional cloning of viruses encoding tumor rejection antigens for recurrent B16tk melanomas. LN/splenocyte cultures (104/well) from mice cured of B16tk recurrent tumors by a combination of GCV and GEEL (as in b) were screened for secretion of IL-17 induced by infection with aliquots of ~104 pfu of the parental GEEL virus stock in the presence of recombinant hsp70 aliquots which contained virus competent for inducing the IL-17 recall response were pooled and expanded in BHK cells (24–36 hours).7 New LN/splenocyte cultures from GEEL-vaccinated mice were infected with serial dilutions of this expanded stock in the presence of recombinant hsp70 and assayed for IL-17 production. The highest dilution of the virus stock which induced IL-17 at levels significantly above background was amplified by passaging through BHK cells for 24–36 hours. Serial dilutions of this expanded stock were screened for their ability to induce IL-17. Ten microliters of aliquots of the highest dilution of the virus which induced IL-17 were used as the starting point for limiting dilution cloning on BHK cells to identify the dilution at which a single virus particle generated cytopathic effect (+).