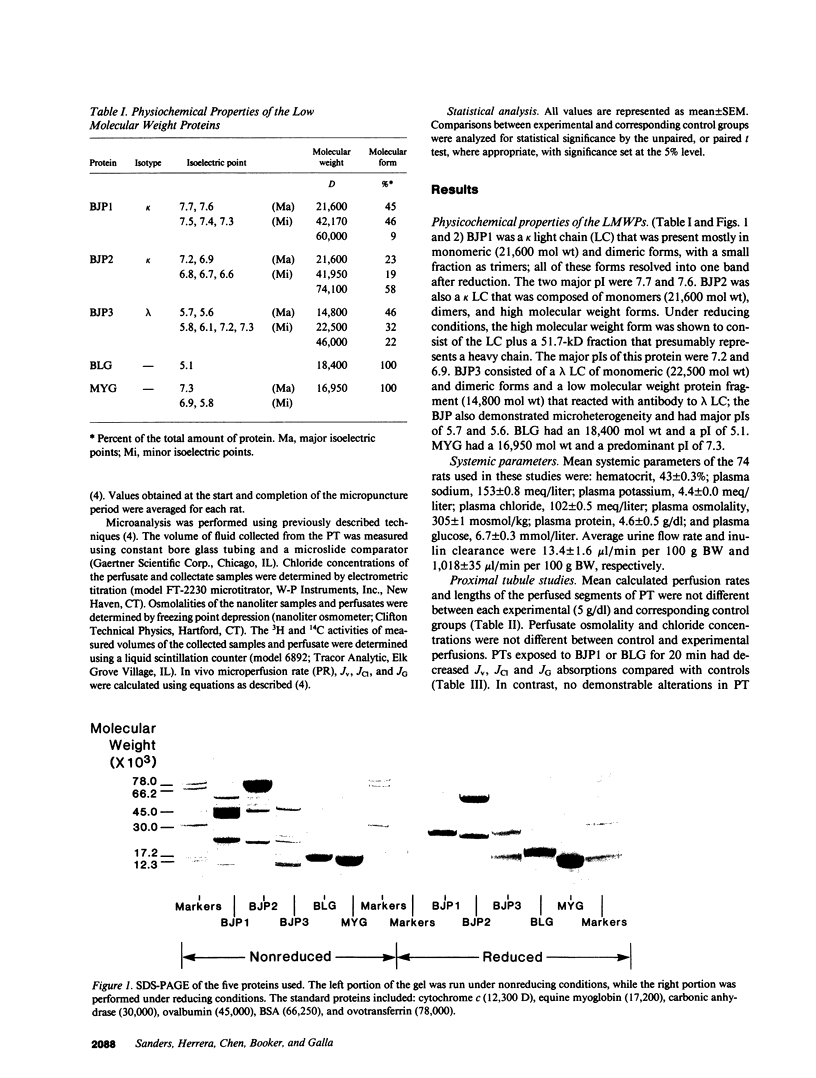
Abstract
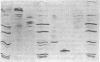
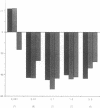
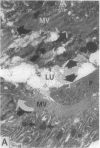
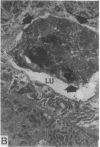
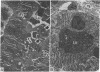
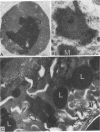
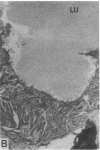
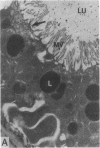
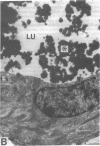
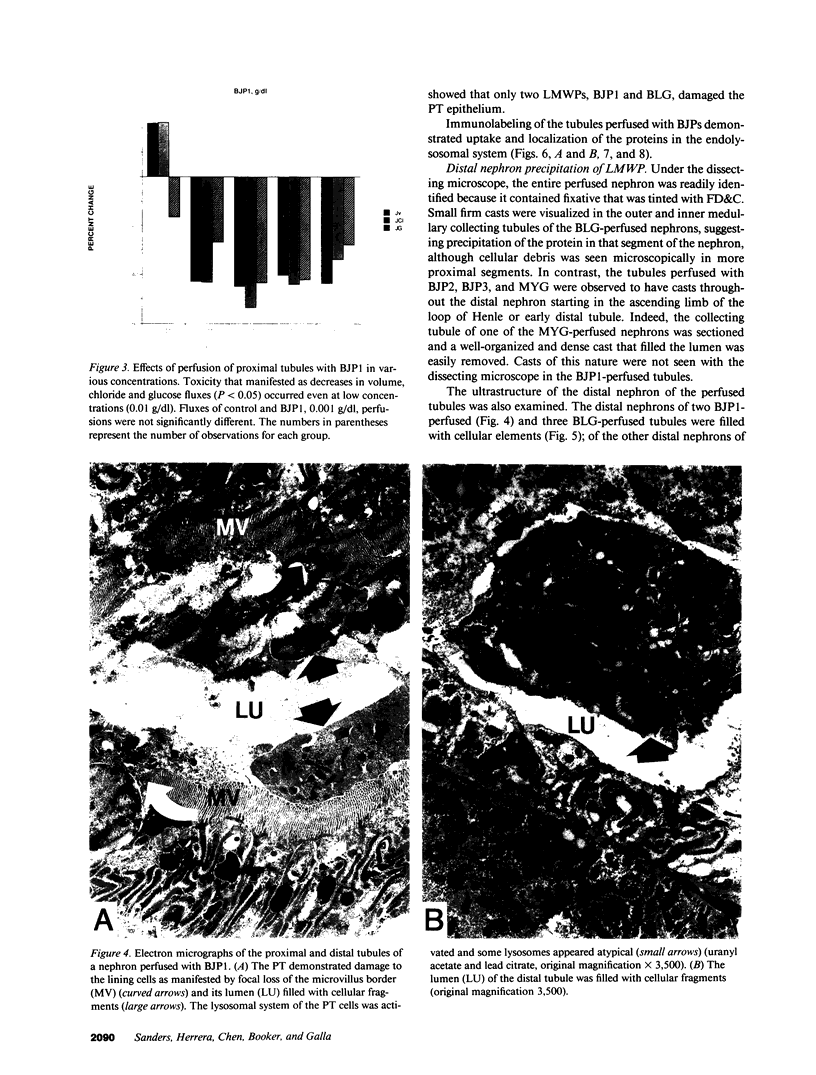
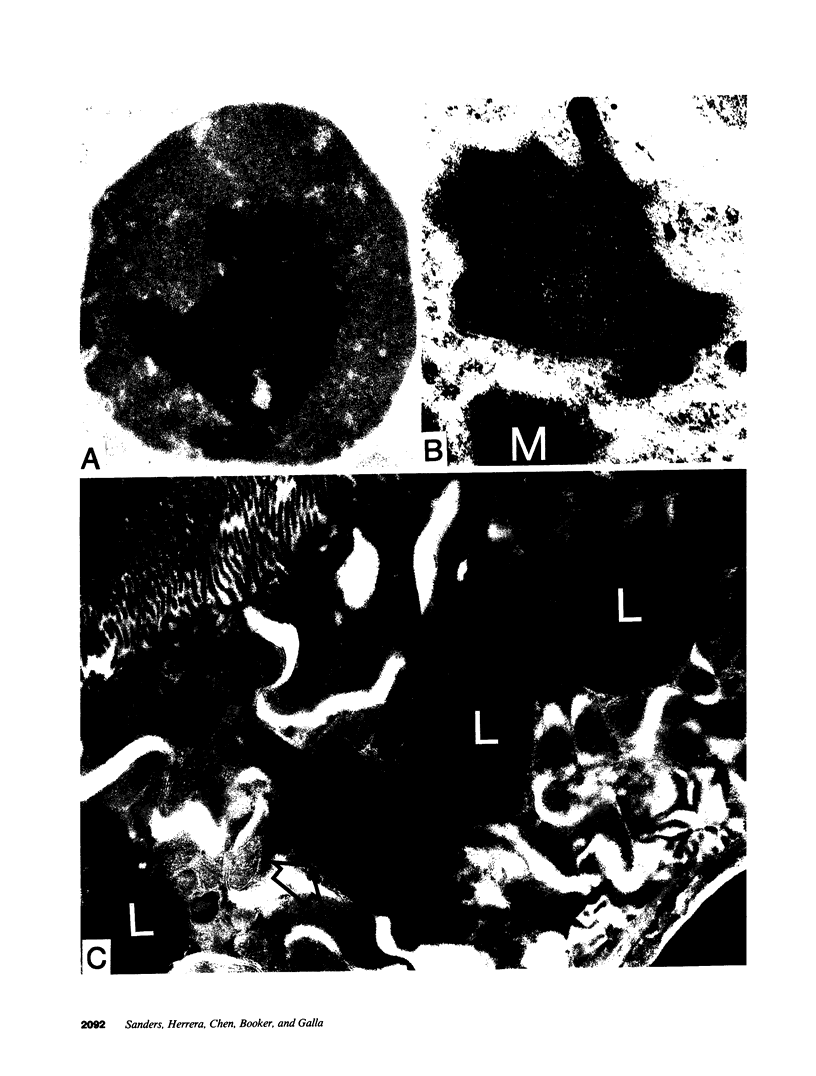
To investigate the pathogenetic mechanisms of tubule nephrotoxicity of low molecular weight proteins (LMWP), proximal tubules (PT) of rats were perfused in vivo with artificial tubule fluid (ATF) containing one of five LMWPs: three human Bence Jones proteins (BJP), beta-lactoglobulin (BLG), and rabbit myoglobin (MYG). Volume (JV), chloride (JCl) and glucose (JG) fluxes in these perfused PTs were compared with those determined using ATF alone. In separate experiments, perfused nephrons were examined with electron and immunoelectron microscopy. After exposure to BJP1 or BLG, JV, JCl, and JG were less (P less than 0.05) than corresponding control fluxes. Cell damage of these perfused PTs, along with cellular debris in the distal tubules, was prominent. The PT lysosomes often appeared atypical and contained crystals. In contrast, perfusion with BJP2, BJP3, or MYG did not alter JV, JCl, or JG. These findings were corroborated by the normal ultrastructure of these PTs despite immunohistochemical evidence of endocytosis of the BJPs. Isoelectric point, molecular form, and isotype were not factors associated with PT damage. In addition, proteins with pI less than 7.4 precipitated in the distal nephron, forming acellular casts. Thus, certain nephrotoxic LMWPs damaged the PT, while others precipitated in the distal tubule, obstructing the nephron. These two pathogenetic mechanisms may independently be responsible for tubulointerstitial nephropathy of LMWPs in humans.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carone F. A., Peterson D. R., Oparil S., Pullman T. N. Renal tubular transport and catabolism of proteins and peptides. Kidney Int. 1979 Sep;16(3):271–278. doi: 10.1038/ki.1979.129. [DOI] [PubMed] [Google Scholar]
- Clyne D. H., Pesce A. J., Thompson R. E. Nephrotoxicity of Bence Jones proteins in the rat: importance of protein isoelectric point. Kidney Int. 1979 Sep;16(3):345–352. doi: 10.1038/ki.1979.137. [DOI] [PubMed] [Google Scholar]
- Cojocel C., Dociu N., Baumann K. Early nephrotoxicity at high plasma concentrations of lysozyme in the rat. Lab Invest. 1982 Feb;46(2):149–157. [PubMed] [Google Scholar]
- Coward R. A., Delamore I. W., Mallick N. P., Robinson E. L. The importance of urinary immunoglobulin light chain isoelectric point (pI) in nephrotoxicity in multiple myeloma. Clin Sci (Lond) 1984 Feb;66(2):229–232. doi: 10.1042/cs0660229. [DOI] [PubMed] [Google Scholar]
- Cushner H. M., Barnes J. L., Stein J. H., Reineck H. J. Role of volume depletion in the glycerol model of acute renal failure. Am J Physiol. 1986 Feb;250(2 Pt 2):F315–F321. doi: 10.1152/ajprenal.1986.250.2.F315. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Cooke C. R., Wright J. R., Humphrey R. L. Renal function in patients with multiple myeloma. Medicine (Baltimore) 1978 Mar;57(2):151–166. doi: 10.1097/00005792-197803000-00003. [DOI] [PubMed] [Google Scholar]
- Defronzo R. A., Humphrey R. L., Wright J. R., Cooke C. R. Acute renal failure in multiple myeloma. Medicine (Baltimore) 1975 May;54(3):209–223. doi: 10.1097/00005792-197505000-00003. [DOI] [PubMed] [Google Scholar]
- Herrera G. A., Turbat-Herrera E. A., Viale G., dell'Orto P., Lott R. L., Colombi R., Alexander R. W., Coggi G. Ultrastructural immunolabeling in renal diseases. Past, present and future expectations. Pathol Immunopathol Res. 1987;6(1):51–63. doi: 10.1159/000157041. [DOI] [PubMed] [Google Scholar]
- Holland M. D., Galla J. H., Sanders P. W., Luke R. G. Effect of urinary pH and diatrizoate on Bence Jones protein nephrotoxicity in the rat. Kidney Int. 1985 Jan;27(1):46–50. doi: 10.1038/ki.1985.8. [DOI] [PubMed] [Google Scholar]
- Johns E. A., Turner R., Cooper E. H., Maclennan I. C. Isoelectric points of urinary light chains in myelomatosis: analysis in relation to nephrotoxicity. J Clin Pathol. 1986 Aug;39(8):833–837. doi: 10.1136/jcp.39.8.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss M. N., Pirani C. L., Osserman E. F. Experimental Bence Jones cast nephropathy. Lab Invest. 1976 Jun;34(6):579–591. [PubMed] [Google Scholar]
- MCINTIRE K. R., POTTER M. STUDIES OF THIRTY DIFFERENT BENCE JONES PROTEIN-PRODUCING PLASMA CELL NEOPLASMS IN AN INBRED STRAIN OF MOUSE. J Natl Cancer Inst. 1964 Oct;33:631–648. [PubMed] [Google Scholar]
- Maack T., Johnson V., Kau S. T., Figueiredo J., Sigulem D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: a review. Kidney Int. 1979 Sep;16(3):251–270. doi: 10.1038/ki.1979.128. [DOI] [PubMed] [Google Scholar]
- Mason J., Olbricht C., Takabatake T., Thurau K. The early phase of experimental acute renal failure. I. Intratubular pressure and obstruction. Pflugers Arch. 1977 Aug 29;370(2):155–163. doi: 10.1007/BF00581689. [DOI] [PubMed] [Google Scholar]
- Park C. H., Maack T. Albumin absorption and catabolism by isolated perfused proximal convoluted tubules of the rabbit. J Clin Invest. 1984 Mar;73(3):767–777. doi: 10.1172/JCI111270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce A. J., Clyne D. H., Pollak V. E., Kant S. K., Foulkes E. C., Selenke W. M. Renal tubular interactions of proteins. Clin Biochem. 1980 Oct;13(5):209–215. doi: 10.1016/s0009-9120(80)80025-7. [DOI] [PubMed] [Google Scholar]
- Rota S., Mougenot B., Baudouin B., De Meyer-Brasseur M., Lemaitre V., Michel C., Mignon F., Rondeau E., Vanhille P., Verroust P. Multiple myeloma and severe renal failure: a clinicopathologic study of outcome and prognosis in 34 patients. Medicine (Baltimore) 1987 Mar;66(2):126–137. doi: 10.1097/00005792-198703000-00004. [DOI] [PubMed] [Google Scholar]
- Ruiz-Guiñazú A., Coelho J. B., Paz R. A. Methemoglobin-induced acute renal failure in the rat. In vivo observation, histology and micropuncture measurements of intratubular and postglomerular vascular pressures. Nephron. 1967;4(5):257–275. doi: 10.1159/000179587. [DOI] [PubMed] [Google Scholar]
- Sanders P. W., Herrera G. A., Galla J. H. Human Bence Jones protein toxicity in rat proximal tubule epithelium in vivo. Kidney Int. 1987 Dec;32(6):851–861. doi: 10.1038/ki.1987.286. [DOI] [PubMed] [Google Scholar]
- Sanders P. W., Herrera G. A., Lott R. L., Galla J. H. Morphologic alterations of the proximal tubules in light chain-related renal disease. Kidney Int. 1988 Apr;33(4):881–889. doi: 10.1038/ki.1988.80. [DOI] [PubMed] [Google Scholar]
- Sanders P. W., Volanakis J. E., Rostand S. G., Galla J. H. Human complement protein D catabolism by the rat kidney. J Clin Invest. 1986 Apr;77(4):1299–1304. doi: 10.1172/JCI112434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolens P., Barnes J. L., Stein J. H. Effect of chronic administration of different Bence Jones proteins on rat kidney. Kidney Int. 1986 Dec;30(6):874–882. doi: 10.1038/ki.1986.267. [DOI] [PubMed] [Google Scholar]
- Smolens P., Venkatachalam M., Stein J. H. Myeloma kidney cast nephropathy in a rat model of multiple myeloma. Kidney Int. 1983 Aug;24(2):192–204. doi: 10.1038/ki.1983.144. [DOI] [PubMed] [Google Scholar]
- Tan M., Epstein W. Polymer formation during the degradation of human light chain and Bence-Jones proteins by an extrct of the lysosomal fraction of normal human kidney. Immunochemistry. 1972 Jan;9(1):9–16. doi: 10.1016/0019-2791(72)90278-9. [DOI] [PubMed] [Google Scholar]
- Weiss J. H., Williams R. H., Galla J. H., Gottschall J. L., Rees E. D., Bhathena D., Luke R. G. Pathophysiology of acute Bence-Jones protein nephrotoxicity in the rat. Kidney Int. 1981 Aug;20(2):198–210. doi: 10.1038/ki.1981.122. [DOI] [PubMed] [Google Scholar]