Figure 2.
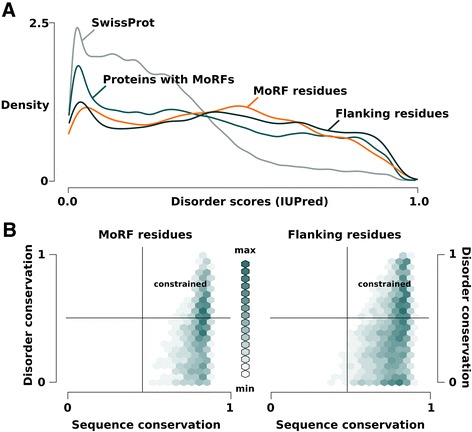
Analyzing MoRFs with DisCons. MoRFs are known to be enriched in disordered residues. Panel A shows normalized density distributions of MoRF regions, MoRF-flanking segments, full-length sequences of the MoRF-containing proteins and all proteins from Swiss-Prot. In these distributions, the area under each curve adds up to one. MoRF residues (orange) are predicted to be more disordered on average than the proteins they are found in, and especially more than the proteins of UniProt/SwissProt. The flanking regions (dark green) of the MoRFs also have significantly higher disorder content, compared to the full-length proteins they are found in. Panel B shows the combined disorder- and sequence conservation scores, which range from 0 (not conserved) to 1 (conserved at all positions in the multiple sequence alignment). By comparing these residue-specific score pairs, DisCons further supports the idea that the sequences of MoRFs (left) are more conserved than that of their flanking regions (right); however, even in the flanking regions intrinsic disorder as a feature is highly conserved, indicating that these segment are required to be flexible in order for the protein segment to function.
