Figure 5.
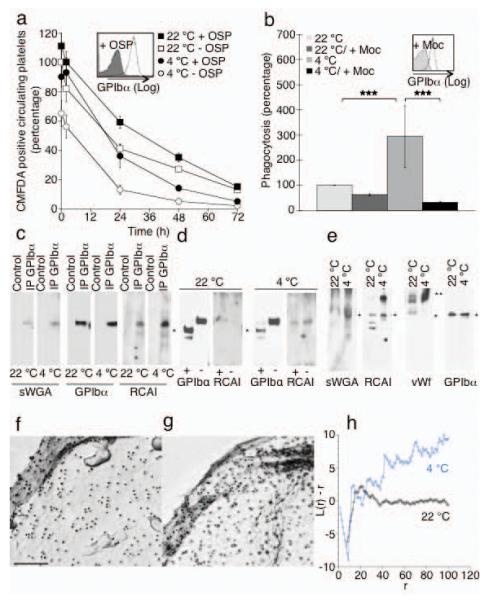
The extracellular domain of GPIbα is required for recognition of platelets by the Ashwell receptor. (a) Survivals of fresh (22 °C) or long-term refrigerated mouse platelets (4 °C) stripped of GPIbα 45 kDa domain by enzymatic treatment with O-sialoglycoprotein endopeptidase (+ OSP). Mean ± s.e.m. for 5-6 recipient mice. Inset. Flow cytometric analysis of GPIbα on untreated (− OSP) and treated (+ OSP) platelets. (b) Effect of the GPIbα N-Terminus removal by mocarhagin (Moc, inset) on the ingestion of fresh (22 °C) or long-term refrigerated human platelets (4 °C) by HepG2 cells. ***P < 0.001 (c) GPIbα immunoprecipitates (IP GPIbα) from fresh (22 °C) or long-term refrigerated mouse platelets (4 °C) were subjected to immunoblotting using RCA I or sWGA lectins. n = 3. (d) GPIbα immunoprecipitates from control platelets (−) or following treatment with OSP (+) were subjected to immunoblotting using anti GPIbα antibodies or RCA I lectin. GPIbα’s C-Terminus is indicated (*). (e) Immunoblotts of total platelet lysates from fresh (22 °C) or long-term refrigerated platelets (4 °C) using sWGA, RCA I lectins or antibodies to mouse vWf or GPIbα. GPIbα (*) and VWf (**) are indicated. n = 3. (f-h) GPIbα aggregates on human cold stored platelets. GPIbα is visualized by electron microscopy. Surface of a (f) fresh platelet (22 °C) and a (g) long-term refrigerated platelet (4 °C). (h) Quantification of gold aggregate size in control and stored platelets. A plot of L(r) - r vs. r visualizes clustering. Values <-1 indicate dispersal whereas values >1 indicate significant clustering.
