Abstract
Multiple distinct epithelial domains are found throughout the airway that are distinguishable by location, structure, function, and cell-type composition. Several progenitor cell populations in the proximal airway have been identified to reside in confined microenvironmental niches including the submucosal glands (SMGs), which are embedded in the tracheal connective tissue between the surface epithelium and cartilage, and basal cells that reside within the surface airway epithelium (SAE). Current research suggests that regulatory pathways that coordinate development of the proximal airway and establishment of progenitor cell niches may overlap with pathways that control progenitor cell responses during airway regeneration following injury. SMGs have been shown to harbor epithelial progenitor cells, and this niche is dysregulated in diseases such as cystic fibrosis. However, mechanisms that regulate progenitor cell proliferation and maintenance within this glandular niche are not completely understood. Here we discuss glandular progenitor cells during development and regeneration of the proximal airway and compare properties of glandular progenitors to those of basal cell progenitors in the SAE. Further investigation into glandular progenitor cell control will provide a direction for interrogating therapeutic interventions to correct aberrant conditions affecting the SMGs in diseases such as cystic fibrosis, chronic bronchitis, and asthma.
Keywords: STEM CELLS, TRACHEA, RESPIRATORY EPITHELIUM, SUBMUCOSAL GLANDS, Wot SIGNALING
The respiratory airway is organized into several physiologically distinct trophic units including the trachea, bronchi, bronchioles, and alveoli. Each trophic unit possesses a specialized epithelium that performs a specific function and has a particular cell-type composition. The proximal trachea is lined with a pseudostratified columnar epithelium that serves as a physical barrier and is composed of cells suited to remove inhaled particles through mucociliary clearance, which involves the production of mucous and serous fluid and its directed movement via motile cilia. Embedded within the connective tissue between the surface epithelium and cartilage are submucosal glands (SMGs), which secrete serous fluids and mucus that moisten and disinfect the inner lining of the trachea and bronchi. The serous fluid derived from SMGs contains numerous antimicrobials such as lysozyme, lactoferrin, and lactoperoxidase that protect the airways from infection. In mice, SMGs are restricted to the proximal portion of the trachea; however, many larger mammals including humans possess SMGs throughout all cartilaginous airways (i.e., trachea and bronchi).This review will focus on the proximal tracheal epithelium with a particular emphasis on epithelial progenitor cells within SMGs and surface airway epithelium (SAE) during development and regeneration. The rationale for reviewing epithelial progenitors during development of the airway is its potential relevance to biologic processes that control progenitor cell niches in the adult airway.
In this review, a progenitor is broadly defined as any relatively undifferentia ted cell that is capable of proliferation and differentiation. However, there is evidence in the airway and other tissues that progenitor cell populations are heterogeneous, and individual progenitors vary in their potential to proliferate and differentiate [Beers and Morrisey, 2011; Wansleeben et al., 2012]. A stem cell is a rare subtype of progenitor that is capable of sustained proliferation and multipotent differentiation. In contrast, many adult progenitors are transient amplifying cells, which proliferate extensively to establish terminally differentiated cells, but have a limited life span in comparison to stem cells. Classical models of progenitor cell proliferation in adult epithelia holds that at steady state, stem cells remain quiescent for the majority of their life-span and infrequently divide asymmetrically to self renew and produce transient amplifying cells that impart the bulk of regeneration through exhaustive cell division [Bertoncello and McQualter, 2013]. However, some in the field have described non-canonical regenerative stem cells called facultative progenitors. These facultative progenitors have been described in the literature as lineage-committed cells that undergo dedifferentiation before proliferating and can in some cases establish multipotent stem cells [Cole et al., 2010; Tata et al., 2013]. However, the same term has also been used to describe any reserve or emergency stem cell population that is involved in regeneration only following extreme injury. It is clear that the airway utilizes different mechanisms of regeneration depending on the extent of injury, and multipotent stem cells often only engage in regeneration following extreme injury [Giangreco et al., 2009]. Recently, analysis of clonal expansion of human airway epithelial cells has shown that airway basal stem cell populations are maintained by stochastic symmetric and asymmetric cell division [Teixeira et al., 2013). Other studies of lineage tracing suggest more directed lineage relationships in the human proximal airway with a subsets of basal cells having multipotent capacity for differentiation into SAE and SMG, and a number of other progenitors with limited capacity for differentiation and proliferation [Engelhardt et al., 1995]. Thus, during development, homeostatic tissue maintenance, and regeneration, different tissue-specific progenitor populations or signaling events may be utilized to generate and renew differentiated cells within the proximal airway epithelium.
Maintaining genomic integrity is of paramount importance for long-lasting progenitor cells. In addition to infrequent and asymmetric cell division, airway progenitors including stem cells have evolved intrinsic cellular properties to protect them against mutation and damage. Studies of distal airway epithelial regeneration have demonstrated that variant club (Clara) cell progenitors are resistant to chemical toxicity from naphthalene and bleomycin [Chen et al., 2012]. Basal cell progenitors have relatively high telomerase activity compared to non-basal columnar cells in the human nasal airway epithelium [Hajj et al., 2006]. In addition, a highly clonogenic side population of Hoechst-excluding human airway epithelial progenitors are enriched with the ATP-binding cassette transporter, ABCG2 (Bcrp-1), which may help protect these cells from membrane-permeable toxins in vivo [Hackett et al., 2008]. Furthermore, elevated aldehyde dehydrogenase activity in proximal airway epithelial stem cells may serve to protect against DNA damage caused by aldehydes from endogenous or exogenous sources [Ghosh et al., 2011b; Hegab et al., 2014). Those studying cancer initiation have also promoted the hypothesis that airway-specific progenitors possess inherent defenses against damage. Based on these drug resistant tendencies, some in the field suspect lung cancers result from pathological changes in the microenvironment of airway stem cells [Succony and Janes, 2014). Although the existence of "cancer stem cells" is still controversial, this area merits further study as many findings indicate that airway progenitor cells possess cell-specific defensive mechanisms that may give them an evolutionary advantage during tumor progression. Interrogating intrinsic properties of airway stem cells may also yield insight into mechanisms affecting the usefulness of pharmacological treatments in the airway.
In addition to having specific intrinsic properties, airway stem cells reside within regulated microenvironmental niches. By being segregated into spatially confined niches, stem cells can be protected from the external environment and are more precisely regulated at homeostasis and following airway injury. Several niches have been identified in the proximal airway of mice, including tracheal SMGs [Borthwick et al., 2001; Engelhardt, 2001; Gomperts and Strieter, 2007; Liu and Engelhardt, 2008; Xie et al., 2011) and intercartilaginous zones of the SAE [Hong et al., 2004b; Cole et al., 2010; Wansleeben et al., 2012]. Generally, niches promote stem cell quiescence unless extensive epithelial cell death in neighboring compartments causes the niche to stimulate stem cell proliferation. In this review, we will explore what is known about developmental processes that control the establishment of proximal airway stem cell niches, how these niches regulate their resident stem cell populations, and relationships between different niches in the proximal airway. Understanding extrinsic processes controlling progenitor cell survival and proliferation within the airway is essential for developing novel therapeutic approaches that target stem cells including regenerative medicine, cell therapy, and gene therapy.
AIRWAY GLAND DEVELOPMENT
One pool of regenerative airway stem cells is maintained in SMGs. Processes that control the development of glands may also reveal biologic signals that influence the establishment of the glandular stem cell niche and suggest how glandular stem cells are regulated. In several animals including rodents, rhesus monkeys, ferrets, pigs, sheep, and humans, developing glands progress through five morphologically distinct stages. In stage I, glandular stem cells coalesce to form primordial glandular placodes (PGPs), which begin to elongate into the mesenchyme. By stage II, the glandular epithelium has formed a lumen contiguous with the conducting airway. By stage III, glands bifurcate laterally and by stage IV form several branching ducts. Finally, SMGs establish a network of differentiated grape-like clusters of secretory acini by stage V [Ritchie et al., 2001]. Glandular development has been most extensively studied in mice where placodes initiate in the nasal epithelium at E14-15 and in the tracheal epithelium within the first day after birth [Driskell et al., 2007]. In contrast, human glandular development initiates within the second trimester of gestation [Thurlbeck et al., 1961]. In addition to this temporal difference in development, SMG abundance and location varies among animal models and humans. For example, in human, ferret, and other large animal models SMGs are found throughout the trachea and bronchi, whereas in rodents SMGs are restricted to the trachea and are largely restricted to the proximal three tracheal rings in mice [Liu et al., 2004]. Initial stages of glandular development may be particularly relevant to understanding glandular stem cells and their niches, since early multipotent glandular stem cells are found within the PGP.
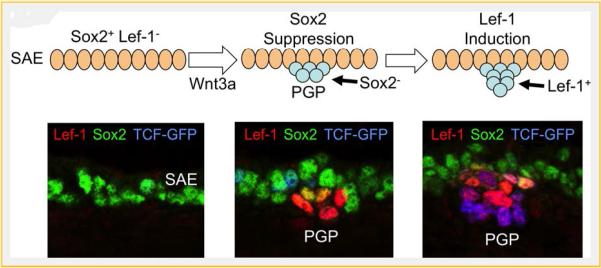
Developmental processes that control transcriptional regulation at glandular initiating stages may provide insights into regulatory pathways that control proliferation of adult glandular progenitors following airway injury. For instance, several studies have demonstrated that canonical Wnt signaling plays a necessary role in both glandular development and proximal progenitor cell fate. Lymphoid enhancer-binding factor 1 (Lef-1), a transcription factor important in canonical Wnt signaling and the transcriptional induction of Cyclin D1 [Shtutman et al., 1999], is required for SMG development in mice [Duan et al., 1999; Driskell et al., 2007]. PGPs of Lef-1knockout mice lack expression of Cyclin D1 and fail to developnormally, suggesting that Lef-1 induction in the PGP is important to drive cell cycle progression during the formation of SMGs [Driskell et al., 2007]. Canonical Wnt ligands, Wnt-1 and Wnt-3a, enhance the transcription of the Lef-1 promoter through the binding of Wnt-induced TCF/β-catenin complexes at multiple sites on the promoter [Filali, 2002; Li et al., 2006; Driskell et al., 2007; Xie et al., 2014]. More recently, studies have shown that Lef-1 transcription is modulated by Sox (SRY-related HMG box) family transcription factors, Sox2 and Soxl7, during early PGP formatio n [Liu et al., 2010; Xie et al., 2014]. Canonical Wnt signals confined to the PGP promote Lef-1 transcription, while in the SAE Sox2 and Sox 17 repress Lef-1transcription. It is currently hypothesized that a localized Wnt signal in the region of the developing PGP down-regulates Sox expression while up-regulating Lef-1 transcription in primordial glandular stem cells [Xie et al., 2014] (Fig. 1). Thus, Wnt signaling is tightly regulated during proximal airway development and plays a pivotal role in glandular stem cell lineage specification and proliferation. This developmental process may inform our understanding of how adult proximal airway progenitors are regulated during homeostasis and/or regeneration.
Fig.1.
Wnt-mediated suppression of Sox2 and induction of lef-1 in postnatal submucosal gland stem cells is critical for tracheal gland formation in mice. At birth, the mouse tracheal surface airway epithelium (SAE) expresses Sox2 [Xie et al., 2014] and Sox17 (not shown) [Liu et al., 2010]. In this context, Sox proteins repress Lef-1 expression by binding to the lef-1 promoter. Local Wnt3A signals at sights of primordial glandular placode (PGP) formation are required for gland development [Driskell etal., 2007]. This Wnt signal leads to at least two key functional changes in PGPs that are required for specification and proliferation of glandular stem cells. First, the Wnt signal suppresses Sox expression in the primordial glandular stem cells [Liu et al., 2010; Xie et al., 2014]. Sox proteins compete for binding at TCF consensus sequences in the lef-1 promoter, thus reduced Sox expression in glandular stem cells primes the Lef-1 promoter for induction by Wnt signaling [Liu et al., 2010; Xie et al., 2014]. Second, Wnt induction raises the level of transcriptionally active β-catenin in glandular stem cells leading to the transcriptional activation of the Lef-1 promoter through the binding of TCF/β-catenin complexes [Liu et al., 2010; Xie et al., 2014]. The figure shows a schematic of early stage I PGP formation and corresponding phenotypic expression of Lef-1 (red), Sox2 (green), and Wnt activation (blue) by immunofluorescence. Wnt activation is indexed by a transgenic reporter line that express an EGFP-histone-28 fusion protein under the direction of a TCF-responsive promoter [Ferrer-Vaquer et al., 2010].
THE PROXIMAL AIRWAY BASAL CELL PROGENITOR
Basal cells are the most thoroughly characterized progenitors of the proximal airway, but questions about their intrinsic and extrinsic cell biology remain. Utilizing several phenotypic markers to perform genetic in vivo lineage tracing experiments and/or in vitro colony forming assays, several groups have demonstrated that basal cells of the tracheal epithelium are multipotent stem cells [Hong et al., 2004b; Hajj et al., 2006; Hackett et al., 2008; Rock et al., 2009; McQualter et al., 2010; Ghosh et al., 2011b, 2013a,b; Hegab et al., 2014] (Fig. 2). However, the functional importance of many of these phenotypic markers to the biology of basal cell progenitors remains unclear, and further investigation into mechanisms of basal cell proliferation, migration, and differentiation may inform how changes in basal cell phenotype influence function in this diverse progenitor population. For example, only a subset of keratin 5 (Krt5) positive basal cells express keratin 14 (Krtl4) at steady state in mice, but after naphthalene injury Krt14 is expressed in all basal cells, and Basal cells that constitutively express Krt14 are unipotent for basal cell progeny at steady state but are multipotent during regeneration, giving rise to basal, club, and ciliated cells after naphthalene exposure [Ghosh et al., 2011a]. Thus, given that Krt14 expression marks a subset of basal cells at steady state and its expression is induced in basal cells during regeneration, Krt14 may be serving an important function for basal cell progenitors (i.e., for cytoskeletal remodeling). Rock et al. [2011] discovered that Notch signaling is necessary for basal cell progenitors to differentiate into ciliated, club, or goblet cells, and during this differentiation process, basal cells maintain Krt8 expression but reduce p63, podoplanin, and NGFR expression [Rock et al., 2011]. However, unipotent basal cell self-renewal was not dependent on Notch signaling [Rock et al., 2011]. Interestingly, a separate group was able to inhibit primary human bronchial basal cell proliferation in vitro by knocking down p63 expression, and demonstrated–using a basal-like cell line (VA 10)–that p63 is needed for basal cell survival, regeneration, and differentiation [Arason et al., 2014]. Additional effort to understand basal cell biology has been directed toward characterizing its transcriptome. A recent study of healthy human bronchial basal cells reveled 1,161 genes and at least 70 transcription factors that are uniquely expressed in basal cells [Hackett et al., 2011]. Further functional analysis of basal cell progenitors will yield important biological insights and broadly impact regenerative medicine for the lung.
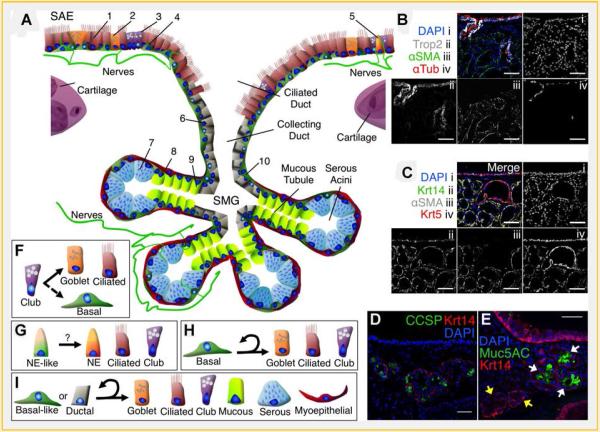
Fig.2.
Structure, function, and cell-type composition of the proximal airway epithelium. A: Within the proximal airway are two anatomically distinct epithelia, the surface airway epithelium (SAE) and the submucosal glands (SMGs). The SAE is composed of ciliated (1), goblet (2), club (3), basal (4), and neuroendocrine (5) cells [Rock and Hogan, 2011]. Ciliated cells (1) possess motile cilia that propel particles out of the airway. Goblet cells (2) secrete mucins that form a protectve barrier and help lubricate the flow of particles. Club cells (3) produce secretog lobins such as CCSP and help detoxify the airway by expressing cytochrome P450 enzymes that oxidize organic compounds. Basal cells (4) reside along the basal lamina and help anchor other cell types of the pseudostratified epithelium. Although neuroendocrine cells (5) are more prevalent inthe distalairways, the proximal airway harbors individual neuroendocrine cells (5) that influence surrounding epithelial cells by secreting neuropeptides such as serotonin, CGRP, and bombesin. SMGs are composed of bundles of serous acini connected to mucous tubules that converge into collecting and ciliated ducts. SMGs harbor ductal (6),serous (7), myoepithelial (8), and mucous (9) cells as well as basal-like cells (1O) similar to basal cells found inthe SAE. Serous cells (7) secrete fluids rich in antimicrobia lenzymes and other peptides that help disinfect the airway and hydrate mucus secreted by glandular mucous cells (9). Contractile myoepithelialcells (8) help excrete glandular secretions. The glands and surface airway epithelium are innervated and neuropept idessuch as CGRP stimulates glandular secretions. B:Trop2 (white, ii) is a calcium signal transducer expressed in the SAE and is expressed in glandular ducts but is not found in other areas of the SMGs. αxSMA (green, iii) marks myoepithelial cells and is not seen in glandular ducts. αxTub (red, iv) marks cilia and is confined to the SAE and glandular ducts. C:Glandular myoepithelial cells express Krtl4 (green, ii),αxSMA (white, iii), and Krt5 (red, iv), but basal-like cells in the ducts do not expressαxSMA (white, iii). D:Clubcell secretory protein (CCSP, green) is expressed in a subset of glandular secretory cells. E:Muc5AC (green) is produced in mucous tubules (white arrowhead) but not serous acini (yellow arrowhead). Scale bars=50 µm. F:A subset of club cells have been shown to proliferate and differentiate into goblet and ciliated cells (solid arrow), but do not self-renew extensively in the proximal airway [Rawlins et al., 2009]. Following basal cell ablation club cells are able to dedifferentiate to regenerate multipotent basal cells (dashed arrow) [Tata et al., 2013]. G:In the distal airways CGRP-expressing neuroendocrine-like progenitors can produce neuroendocrine, club, and ciliated cells following naphthalene exposure [Song et al., 2012] and may also play a regenerative role in the proximal airway. H:Atleast a subset of proximal airway basal cells are self-renewing, multipotent progenitor cells and are able to differentiate into goblet, ciliated and club cell types [Hong et al.,2004b; Hajj et al., 2006; Hackett et al., 2008; Rock et al., 2009; McQualter etal.,2010; Ghosh et al., 2011b, 2013a,b; Hegab etal., 2012, 2014]. I:An epithelial progenitor cell isolated from SMGs has been shown to self-renew and generate several cell types found in the SAE and SMGs in various in vitro and ex vivo assays [Hegab et al., 2011, 2012, 2014]. Data from several studies suggest that this cell type resides within glandular ducts and has a basal-like phenotype [Borthwick et al., 2001; Engelhardt, 2001; Hegabet al., 2011, 2012, 2014], but it is still a relatively uncharacterized progenitor cell.
Indeed, in recent years several subpopulations of basal cells displaying various combinations of phenotypic markers have been shown to clonally produce multiple airway cell types in vitro (Table I). Different fluorescence-activated cell sorting (FACS) strategies enrich or deplete specific cell types based on cell-intrinsic properties using fluorescent reagents or transgenic fluorescence activity, but to our knowledge a direct comparison between cell populations isolated using different strategies has not been performed. A direct comparison of distinct subpopulations of basal cells would provide evidence for or against the existence of a basal cell progenitor hierarchy, a topic that is still currently debated, and address whether a "master stem cell" exists at the apex of this progenitor hierarchy. For example, are subsets of tissue-specific stem cells in the airway fated to remain as stem cells during development, and is this potential retained even when isolated? Does the maintenance of multipotency and unlimited proliferative potential in airway stem cells depend on niche-specific micro-environments or can tissue-specific stem cells adapt to variable conditions to reestablish homeostatic niches? Lineage tracing studies in mouse tracheal and bronchial epithelium using a KRT14-CreER driver suggests that subpopulations of basal cells may have multipotent or unipotent capacities following airway injury [Hong et al., 2004b]. These findings are similar to viral lineage tracing studies with human primary bronchial airway cells seeded into denuded rat tracheal xenograft scaffolds in which a subset of surface airway epithelial (SAE) cells produced large colonies consisting of multiple cell types including SMGs [Engelhardt et al., 1995]. However, clonal expansion of lineage-labeled basal cells in the bronchial epithelium revealed that most clusters of tagged cells lacked basal cells [Hong et al., 2004a], and this may suggest that either bronchial basal cell progenitors have a limited capacity to self-renew or that non-basal, KRT14-expressing progenitors are positioned at a higher tier within the airway stem cell hierarchy [Hong et al., 2004b]. However, other studies have proposed that all basal cells have the capacity for multipotent differentiation and that the fate of the basal cell progenitor is influenced by its local environment and type of injury [Rock et al., 2009]. Most certainly the type and extent of injury will influence the ability of various progenitors to commit down certain lineages, and this is likely also applicable to the type of culture conditions used to assess multipotency in isolated progenitors. Thus, a direct comparison between subpopulations of basal cells within several in vitro and potentially ex vivo (i.e., xenograft) assays may help to delineate specific cell-extrinsic conditions necessary for basal cell expansion and differentiation. Without a doubt, impressive progress has been made in recent years to understand basal cell progenitor phenotypes in the proximal airway, but there is still much to be learned.
TABLE 1.
Examples of FACS Strategies Used to Isolate Basal Cell Progenitors
| Sorting strategy | Assay type | Refs. |
|---|---|---|
| Mouse | ||
| ITGAS; p63; NGFR; Krt5 | 3D sphere-forming assay | Rock et al. [2009] |
| EpCAMhigh ;ITGA6; CD104; CD24low | Organoid-forming co-culture assay | McQualter et al. [2010] |
| ITGA6highSca-1; ALDH | Irradiated fibroblast co-cultures | Ghosh et al. [2011b] |
| H2B-GFPhigh a; [TGA6-Sca-1 | Irradiated fibroblast co-cultures | Ghosh et al. [2013b] |
| TROP2; ITGA6; ALDHhigh | 3D sphere-forming assay | Hegabet al. [2014] |
| Human | ||
| TF;CD151 | ALI culture, xenograft assay | Hajj et al. [2006] |
| Hoescht 33342low; CD45neg | All culture | Hackett et al. [2003] |
| CD44;CD166;ALDHhigh | 3D sphere-forming assay | Hegab et al. [2012] |
| TF; CD151neg | Irradiated fibroblast co-cultures | Ghosh et al. [2013a] |
H2B-GFP retention indicated infrequent in vivo proliferation while dilution indicated frequent division.
Studies focusing on how basal cells are regulated within their microenvironment and how the surface basal cell niche may differ from the SMG progenitor niche are greatly needed. In several approaches, purified basal cell populations require being co-cultured with non-epithelial primary cells or with irradiated fibroblast cells to efficiently proliferate in vitro. These results illustrate the importance of extrinsic factors within the basal cell microenvironment for progenitor cell expansion and differentiation. Recently, work by has shown that a subpopulation of basal cells, which divides infrequently in vivo, is capable of forming rimmed-clones in vitro [Ghosh et al., 2011b]. In this assay, rims demonstrated in vitro niche-like properties by being enriched with slowly cycling cells. The ability of an isolated single multipotent basal cell to form this rimmed niche suggests that stem cells in the proximal airway may have the ability to form their own microenvironment required for maintenance ofstemness [Ghosh et al., 2011b]. These findings are especially encouraging to the cell therapy field, since establishment of stem cell niches in the airway have been long thought to be dependent on multiple unique cell types. Interestingly, these clonal rimmed niches express canonical Wnt/β-catenin signals [Ghosh et al., 2013b], suggesting a Wnt-dependent transcriptional profile within the niche likely regulates the basal cell progenitor. Indeed, precise control of Wnt/β-catenin signaling is needed during proliferation and differentiation phases of establishing primary epithelial air-liquid interface cultures and Wnt/β-catenin signaling is induced in the tracheal epithelium shortly after naphthalene injury of mice [Brechbuhl et al., 2011]. An independent study found that increased TOPgal expression correlated with regeneration fellew polidocanol injury and transgenic stabilization of transcriptionally active β-catenin led to increased basal cell proliferation and when sustained Jed to a preinvasive squamous Jung cancer phenotype [Giangreco et al., 2012]. It is unclear if factors stimulating Wnt/β-catenin signaling originate from basal stem cells themselves or if other cells are the source.
Niche-specific paracrine pathways, such as Wnt/β-catenin signaling, control progenitor cell fate during development, homeostasis, and regeneration [Wansleeben et al., 2012] and likely help coordinate the interplay between distant niche compartments in vivo. For example, it has been suggested that SMGs are a protected niche for the airway epithelium and may serve as a facultative niche for the surface airway following severe injury (Fig. 2) [Borthwick et al., 2001; Engelhardt, 2001; Gomperts and Strieter, 2007; Xie et al., 2011]. Thus different injury models may present differing results in regards to progenitor cells and their behavior. Where stimulatory or inhibitory paracrine factors originate in the proximal airway is still unknown, and future work to identify the origins of these signals will accelerate our understanding of the development of disease and thereby its prevention.
THE SUBMUCOSAL GLAND (SMG) PROGENITOR CELL NICHE
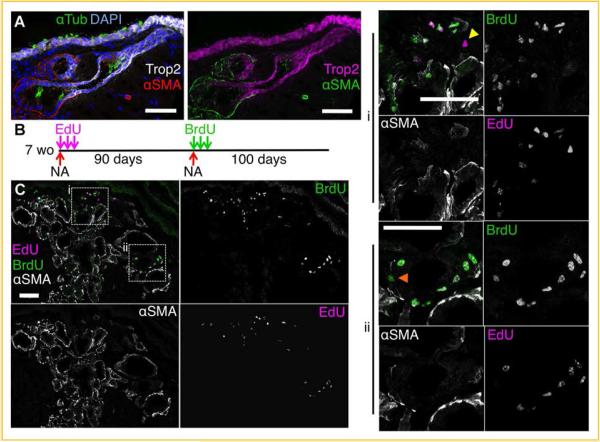
In addition to basal cells in the SAE, research has revealed that SMGs also contain slowly cycling proximal airway epithelial progenitor cells (Fig. 2) [Borthwick et al., 2001; Engelhardt, 2001; Hegab et al., 2011, 2012; Xie et al., 2011]. After naphthalene injury, slowly cycling progenitors expand and then re-enter a quiescent state (Fig. 3). Mitotic cells can be labeled during this regeneration phase with nucleotide analogues, which are incorporated into the DNA of dividing cells. Minimally expanding progenitors retain this label, while more mitotic transient amplifying cells dilute the label beyond a detectable level. Label-retaining cells are enriched in regions of the SMGs including the ducts (Fig. 3). Importantly, these SMG progenitors can re-enter the cell cycle following repeated injury, as detected using two nucleotide analogs but remain slowly cycling (Fig. 3B,C). Given that progenitor cells within the SMG niche are dysregulated in diseases such as cystic fibrosis [Xie et al., 2011], understanding SMG structure and function at steady state and following injury is important to understand disease progression in the airway. SMGs are a specialized secretory epithelium with a grape-like structure consisting of serous-producing acini connected to mucous-producing tubules, which merge into collecting and ciliated ducts to excrete their contents into the airway lumen (Fig. 2A) [Liu and Engelhardt, 2008]. The extensive surface area of the glandular epithelium provides abundant contact with mesenchymal cell types, which play an important regulatory role during glandular development and likely during injury-induced or homeostatic regeneration .
Fig.3.
Slowly cycling SMG progenitor cells reside in clusters with in SMG ducts. A:αSMA is expressed in glandular serous acini and mucous tubules but is not expressed in ductal epithelia of the glands. Trop2 is expressed in the surface airway epithelium and in gland ducts, but is not expressed in serous acini or mucous tubules. B:Seven-week-old mice were injured with a single i.p. injection of naphthalene (NA) (300µg/g bodyweight) and dividing cells were labeled with three i.p. injections of EdU (25µg/g bodyweight) over 6 days. The mice were allowed to recover from this initial injury for 90 days, during which time dividing cells diluted the initial EdU label. Mice were injured again with an i.p. injection of naphthalene (300µg/g bodyweight) and dividing cells were labeled with three i.p. injections of BrdU (50µg/g bodyweight) over 6 days. Mice were allowed to recover for 100 days, during which time dividi cell diluted both nucleotide labels. C: Each of the nucleotides were then localized together with αSMA using either antibody staining (for BrdU) or with a chemical ion (for EdU). Label-retaining cells (LRCs) are cells that replicated and incorporated labeling nucleotide analogues from the pulses after injury, but subsequently remained relatively quiescent. Cells that continued to divide diluted the nucleotide analogue(s) beyond a detectable level. Nucleotide double-positive LRCs are progenitors that re-entered the cell cycle during the second regeneration phase and re-established a quiescent phenotype. A representative photomicrograph shows LRCs and recycling LRCs localized in clusters within glandular ducts that are αSMA-negative. Arrowheads mark single EdU-positive LRCs (yellow) or BrdU-positive LRCs (orange). The majority of LRCs are unmarked and double positive for EdU and BrdU. All scale bars= 50µm, and all single channel panels are the same scale as their respective merged panels.
Furthermore, neuronal input regulates glandular secretions and can promote progenitor cell proliferation or quiescence [Xie et al., 2011]. The neuropeptide, cakitonin gene-related peptide (CGRP) activates glandular secretions by stimulating the chloride ion channel, cystic fibrosis transmembrane conductance regulator (CFTR), and a CFTR deficiency in human, ferret, pig, and mouse airways leads to a chronic compensatory elevation of CGRP signals within SMGs. Further interrogation in mice revealed that chronic activation of CGRP in SMGs in the CF disease state leads to dysregulation of the SMG progenitor niche and resulted in depletion of slowly cycling progenitors from the SMGs and a redistribution to the SAE. However, although in vitro and in vivo CGRP over-expression promoted progenitor cell proliferation, components of the CGRP receptor were not robustly expressed on slowly cycling progenitors, which suggests that progenitors likely do not respond to CGRP directly, and an intermediate cell type within the SMG niche may mediate CGRP-dependent progenitor proliferation through an unidentified down-stream signaling pathway [Xie et al., 2011]. This CGRP-dependent pathway that promotes glandular progenitor cell proliferation appears to also be functional in wild-type mice, since airway injury promotes the induction ofCGRP in the SMGs and SAE [Xie et al., 2011]. The specific neuronal pathways that control these responses have yet to be determined, but since CGRP induces SMG secretions, this mechanism likely acts to both protect the airway from infection following injury while also inducing progenitor cell proliferation and repair.
Delineating niche-specific cell types and how they influence progenitor cell responses in the setting of homeostasis and injury is an important opportunity for further research. The glandular epithelium displays many of the phenotypic markers expressed in other cell types and trophic units of the airway epithelium, and thus glands display a greater diversity of cellular phenotypes then in other epithelial compartments. For example, in addition to serous and mucous secretory cells, basal-like cells-marked by their expression of p63, keratin 5, and/or keratin 14-are present in SMGs in addition to α-smooth muscle actin-positive myoepithelial cells, CGRP-positive neuroendocrine-Like cells, and CCSP-positive club-like cells (Fig. 2B,D). However, a large gap in knowledge exists in regards to niche-specific regulation of progenitors including how different ancillary cells within or surrounding the glandular niche interact and influence epithelial progenitor cell proliferation and preservation.
Besides regulation from within the SMG niche, signals from other airway niches likely play a role in SMG stem cell expansion or quiescence. Thus, it has been suggested that SMGs are a facultative stem cell niche for the proximal airway. Certainly, SMGs are more protected from environmental injuries due to their anatomical location in the airway. However, it remains unclear if SMG progenitors serve as a reservoir for the SAE at homeostasis. In part, the answer to this question has been difficult to address since SMGs in the mouse proximal trachea likely serve different functions than in other mammals that possess SMGs throughout the conducting cartilaginous airways.
Observations made from injury-induced regeneration models in mice have Jed the field to propose the idea of a facultative progenitor population as either a cache of reserve multipotent stem cells or a subset of differentiated cells able to either de-differentiate into multipotent stem cells or adopt a more stem cell-like phenotype. At steady state stem cells enter symmetric or asymmetric cell division stochastically and daughter progenitor cells with limited proliferative potential are sufficient to maintain homeostasis [Giangreco et al., 2009; Wansleeben et al., 2012]. But following severe injury, stem cells proliferate in relative synchrony either as a direct response to signals proportional to the severity ofinjury or as a response to the Joss of mitotically limited progenitor cells, which serve as a "first line of defense." Variant club stem cells in the bronchiolar epithelium contributed to regeneration only after severe naphthalene-mediated injury, which was determined by a reduction in body mass greater than 10% [Giangreco et al., 2009]. Additionally, the tracheobronchial epithelium is maintained by secretory cells and basal cells that are either positive or negative for keratin 14 (Krt14), but after naphthalene injury both basal cell populations adopt a Krt14-positive phenotype as they contribute to regeneration [Cole et al., 2010]. Further, plasticity of progenitor cells in the airway was recently demonstrated in a study by Tata et al. [2013] where basal cells where ablated by diphtheria toxin, which prompted differentiated secretory cells to de-differentiate to produce multipotent stem cells [Tata et al., 2013). However, it is largely unknown how different injury signals coordinate unique regenerative responses by airway progenitors.
RELEVANCE TO DISEASE
Pathological remodeling of the proximal airway epithelium is seen in chronic bronchitis, asthma, and cystic fibrosis. Cystic fibrosis results from a monogenetic defect affecting the chloride ion channel, cystic fibrosis transmembrane conductance regulator (CFTR). Inadequate chloride and bicarbonate ion transport in the CF airway results in hyperviscous epithelial secretions and impaired mucociliary clearance, which is a cause of persistent pulmonary infection and chronic inflammation [Rowe et al., 2005). Protracted imbalances in epithelial homeostasis result in elevated levels of repair and cellular proliferation in CF airways [Leigh et al., 1995), and human airways display glandular hypertrophy and hyperplasia, glandular serous to mucous cell metaplasia, and basal and goblet cell hyperplasia [Oppenheimer and Esterly, 1975], which implicates the disruption of normal progenitor cell biology. Airway remodeling in CF, which includes bronchiectasis, likely disturbs the normal homeostatic properties of airway progenitor cell niches in many ways that are yet to be discovered. Whether disruption of these niches, as shown for SMGs [Xie et al., 2011], contribute to disease progression in the CF lung remains to be determined.
Abundant signaling events regulate progenitor cell proliferation and differentiation, and in airway diseases such as cystic fibrosis, chronic bronchitis, and asthma these processes may be dysregulated. Relatively little is known about the various processes that coordinate healthy progenitor cell proliferation, and elucidating normal regenerative mechanisms will aid in drug discovery and the development of novel treatments . Thorough characterization of progenitor cells in their native environment will assist researchers and clinicians with a more precise benchmark for the design of engineered tissues. Researchers will have a more complete idea of how to utilize and culture patient-derived primary progenitor cells in vitro for testing customized pharmacological treatments. By understanding cell-intrinsic characteristics of the airway progenitor, as well as properties of the progenitor cell niche, researchers can begin to address issues that contribute to niche-remodeling in disease.
ACKNOWLEDGMENTS
This work was supported by NIH grants DK047967 (to JFE) and the University of Iowa Center for Gene Therapy (DK54759).
REFERENCES
- Arason AJ, Jonsdottir HR, Halldorsson S, Benediktsdottir BE, Bergthorsson JT, Ingthorsson S, Baldursson 0, Sinha S, Gudjonsson T, Magnusson MK. DeltaNp63 bas a role in maintaining epithelial integrity in aiiway epithelium. PLoS ONE. 2014;9:e88683. doi: 10.1371/journal.pone.0088683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers MF, Morrisey EE. The three Rtapos;s of lung health and disease: Repair, remodeling, and regeneration. J Clio Invest. 2011;121:2065–2073. doi: 10.1172/JCI45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoncello I, McQualter JL. Lung stem cells: Do they exist? Respirology. 2013;18:587–595. doi: 10.1111/resp.12073. [DOI] [PubMed] [Google Scholar]
- Borthwick D, Sbabbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- Brechbubl HM, Ghosh M, Smith MK, Smith RW, Li B, Hicks DA, Cole BB, Reynolds PR, Reynolds SD. β-Catenin dosage is a critical determinant of tracheal basal cell fate determination. Am J Pathol. 2011;179:367–379. doi: 10.1016/j.ajpath.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee J-H, Jiang D, Noble PW, Randell SH, Kim CF, Stripp BR. Airway epithelial progeni tors are region-specific and show differential responses to bleomycin-induced lung injury. Stem Cells. 2012;30:1948–1960. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BB, Smith RW, Jenkins KM, Graham BB, Reynolds PR, Reynolds SD. Tracheal basal cells : A facultative progenitor cell pool. Am J Pathol. 2010;177:362–376. doi: 10.2353/ajpath.2010.090870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Goodheart M, Neff T, Liu X, Luo M, Moothart C, Sigmund CD, Hosokawa R, Chai Y, Engelbardt JF. Wnt3a regulates Lef-1 expression during airway submucosal gland morphogenesis. Dev Biol. 2007;305:90–102. doi: 10.1016/j.ydbio.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Yue Y, Zhou W, Lebed B, Ritchie TC, Grosscbedl R, Engelhardt JF. Submucosal gland development in the airway is controlled by lymphoid enhancer binding factor I (LEFI) Development. 1999;126:4441–4453. doi: 10.1242/dev.126.20.4441. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF. Stem cell niches in the mouse airway. Am J Respir Cell Mol Biol. 2001;24:649–652. doi: 10.1165/ajrcmb.24.6.f206. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Schlossberg H, Yankaskas JR, Dudus L. Progenitor cells of the adult human airway involved in submucosal gland development. Development. 1995;121:2031–2046. doi: 10.1242/dev.121.7.2031. [DOI] [PubMed] [Google Scholar]
- Ferrer-Vaquer A, Piliszek A, Tian G, Aho RJ, Dufort D, Hadjantonakis A-K. A sensitive and bright single-cell resolution live imaging reporter of Wnt/β-catenin signaling in the mouse. BMC Dev Biol. 2010;10:121–139. doi: 10.1186/1471-213X-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filali M. Wnt-3A/β-catenin signaling induces transcription from the LEF-1 promoter. J Biol Chem. 2002;277:33398–33410. doi: 10.1074/jbc.M107977200. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Brecbbuhl HM, Smith RW, Li B, Hicks DA, Titchner T, Runkle CM, Reynolds SD. Context-dependent differentiation of multipotential keratin 14-expressing tracheal basal cells. Am J Respir Cell Mol Biol. 2011a;45:403–410. doi: 10.1165/rcmb.2010-0283OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Helm KM, Smith RW, Giordanengo MS, Li B, Shen H, Reynolds SD. A single cell functions as a tissue-specific stem cell and the in vitro niche-forming cell. Am J Respir Cell Mol Biol. 2011b;45:459–469. doi: 10.1165/rcmb.2010-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Ahmad S, Jian A, Li B, Smith RW, Helm KM, Seibold MA, Groshong SD, White CW, Reynolds SD. Human tracheobroncbial basal cells : Normal versus remodeling/repairing phenotypes in vivo and in vitro. Am J Respir Cell Mol Biol. 2013a;49:1127–1134. doi: 10.1165/rcmb.2013-0049OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Smith RW, Runkle CM, Hicks DA, Helm KM, Reynolds SD. Regulation of trachebronchial tissue-specific stem cell pool size. Stem Cells. 2013b;31:2767–2778. doi: 10.1002/stem.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A, Arwert EN, Rosewell JR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore aiiways after injury. Proc Natl Acad Sci USA. 2009;106:9286–9291. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A, Lu L, Vickers C, Teixeira VH, Groot KR, Butler CR, Ilieva EV, George PJ, Nicholson AG, Sage EK, Watt FM, Janes SM. β-Catenin determines upper airway progenitor cell fate and preinvasive squamous lung cancer progression by modulating epithelial-mesenchymal transition. J Pathol. 2012;226:575–587. doi: 10.1002/path.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts BN, Strieter RM. Stem cells and chronic lung disease. Annu Rev Med. 2007;58:285–298. doi: 10.1146/annurev.med.58.081905.134954. [DOI] [PubMed] [Google Scholar]
- Hackett T-L, Shaheen F, Johnson A, Wadsworth S, Pechkovsky DV, Jacoby DB, Kicic A, Stick SM, Knight DA. Characterization of side population cells from human airway epithelium. Stem Cells. 2008;26:2576–2585. doi: 10.1634/stemcells.2008-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett NR, Sbaykhiev R, Walters MS, Wang R, Zwick RK, Ferris B, Witover B, Salit J, Crystal RG. The human airway epithelial basal cell transcriptome. PLoS ONE. 2011;6:e18378. doi: 10.1371/journal.pone.0018378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajj R, Baranek T, Le Naour R, Lesimple P, Pucbelle E, Coraux C. Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells. 2006;25:139–148. doi: 10.1634/stemcells.2006-0288. [DOI] [PubMed] [Google Scholar]
- Hegab AE, Ha VL, Gilbert JL, Zhang KX, Malkoski SP, Chon AT, Darmawan DO, Bisbt B, Ooi AT, Pellegrini M, Nickerson DW, Gomperts BN. Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells. 2011;29:1283–1293. doi: 10.1002/stem.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegab AE, Ha VL, Darmawan DO, Gilbert JL, Ooi AT, Attiga YS, Bisbt B, Nickerson DW, Gomperts BN. Isolation and in vitro characterization of basal and submucosal gland duct stem/progenitor cells from human proximal airways. Stem Cells Transl Med. 2012;I:719–724. doi: 10.5966/sctm.2012-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegab AE, Ha VL, Bisbt B, Darmawan DO, Ooi AT, Zhang KX, Paul MK, Kim YS, Gilbert JL, Attiga YS, Alva-Ornelas JA, Nickerson DW, Gomperts BN. Aldehyde debydrogenase activity enriches for proximal airway basal stem cells and promotes their proliferation. Stem Cells Dev. 2014;23:664–675. doi: 10.1089/scd.2013.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells:Evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004;286:L643–L649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- Leigh MW, Kylander JE, Yankaskas JR, Boucher RC. Cell proliferation in bronchial epithelium and submucosal glands of cystic fibrosis patients. Am J Respir Cell Mol Biol. 1995;12:605–612. doi: 10.1165/ajrcmb.12.6.7766425. [DOI] [PubMed] [Google Scholar]
- Li TWH, Ting J-HT, Yokoyama NN, Bernstein A, van de Wetering M, Waterman ML. Wot activation and alternative promoter repression of LEFI in colon cancer. Mol Cell Biol. 2006;26:5284–5299. doi: 10.1128/MCB.00105-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Engelhardt JF. The glandular stem/progenitor cell niche in airway development and repair. Proc Am Thorac Soc. 2008;5:682–688. doi: 10.1513/pats.200801-003AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Driskell RR, Engelhardt JF. Airway glandular development and stem cells. Curr Top Dev Biol. 2004;64:33–56. doi: 10.1016/S0070-2153(04)64003-8. [DOI] [PubMed] [Google Scholar]
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, Dapos;Amico M, Pestell R, Ben-Zeaposev A. The cyclin DI gene is a target of the beta-catenin/ LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Yao E, Lin C, Gacayan R, Chen MH, Chuang PT. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci USA. 2012;109:17531–17536. doi: 10.1073/pnas.1207238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Luo M, Xie W, Wells JM, Goodheart MJ, Engelhardt JF. Soxl7 modula tes Wnt3A/beta-catenin-media ted transcriptional activation of the Lef-1 promo ter. Am J Physiol Lung Cell Mol Physiol. 2010;299:L694–L710. doi: 10.1152/ajplung.00140.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci USA. 2010;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer EH, Esterly JR. Pathology of cystic fibrosis review of the literature and comparison with 146 autopsied cases. Perspect Pediatr Pathol. 1975;2:241–278. [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass OM, Auten RL, Hasegawa H, Wang F, Hogan BLM. The role of Scgblal + Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie TC, Zhou W, McKinstry E, Hosch M, Zhang Y, Nathke I, Engelhardt JF. Developmental expression of catenins and associated proteins during submucosal gland morphogenesis in the airway. Exp Lung Res. 2001;27:121–141. doi: 10.1080/019021401750069375. [DOI] [PubMed] [Google Scholar]
- Rock JR, Hogan BLM. Epithelial progeni tor cells in lung development, maintenance, repair, and disease. Ann Rev Cell Dev Biol. 2011;27:493–512. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BLM. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Gao X, Xue Y, Randell Scott H, Kong Y-Y, Hogan Brigid LM. Notch-dependen t differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Succony L, Janes SM. Airway stem cells and lung cancer. QJM. 2014 doi: 10.1093/qjmed/hcu040. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, Medoff BO, Rajagopal J. Dedifferen tiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira VH, Nadarajan P, Graham TA, Pipinikas CP, Brown JM, Falzon M, Nye E, Poulsom R, Lawrence 0, Wright NA, McDonald S, Giangreco A, Simons BO, Janes SM. Stochastic homeostasis in human airway epithelium is achieved by neutral competition of basal cell progenitors. eLife. 2013;2:e00966. doi: 10.7554/eLife.00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlbeck WM, Benjamin B, Reid L. Development and distribution of mucous glands in the foetal human trachea. Br J Dis Chest. 1961;55:54–64. doi: 10.1016/s0007-0971(61)80002-8. [DOI] [PubMed] [Google Scholar]
- Wansleeben C, Barkauskas CE, Rock JR, Hogan BLM. Stem cells of the adult lung : Their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip Rev Dev Biol. 2012;2:131–148. doi: 10.1002/wdev.58. [DOI] [PubMed] [Google Scholar]
- Xie W, Fisher JT, Lynch TJ, Luo M, Evans TIA, Neffil TL, Zhou W, Zhang Y, Ou Y, Bunnett NW, Russo AF, Goodheart MJ, Parekh KR, Liu X, Engelhardt JF. CGRP induction in cystic fibrosis airways alters the submucosal gland progenitor cell niche in mice. J Clin Invest. 2011;121:3144–3158. doi: 10.1172/JCI41857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Lynch TJ, Liu X, Tyler SR, Yu S, Zhou X, Luo M, Kusner OM, Sun X, Yi Y, Zhang Y, Goodheart MJ, Parekh KR, Wells JM, Xue HH, Pevny LH, Engelhardt JF. Sox2 modulates Lef-1 expression during airway submucosal gland development. Am J Physiol Lung Cell Mol Physiol. 2014;306:L645–L660. doi: 10.1152/ajplung.00157.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]