Fig.1.
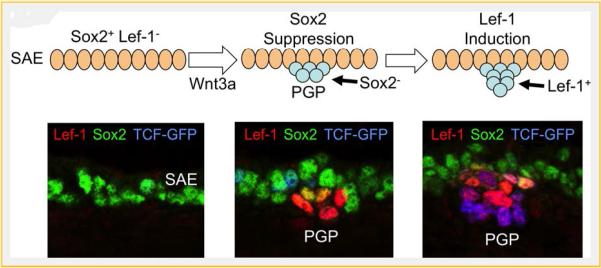
Wnt-mediated suppression of Sox2 and induction of lef-1 in postnatal submucosal gland stem cells is critical for tracheal gland formation in mice. At birth, the mouse tracheal surface airway epithelium (SAE) expresses Sox2 [Xie et al., 2014] and Sox17 (not shown) [Liu et al., 2010]. In this context, Sox proteins repress Lef-1 expression by binding to the lef-1 promoter. Local Wnt3A signals at sights of primordial glandular placode (PGP) formation are required for gland development [Driskell etal., 2007]. This Wnt signal leads to at least two key functional changes in PGPs that are required for specification and proliferation of glandular stem cells. First, the Wnt signal suppresses Sox expression in the primordial glandular stem cells [Liu et al., 2010; Xie et al., 2014]. Sox proteins compete for binding at TCF consensus sequences in the lef-1 promoter, thus reduced Sox expression in glandular stem cells primes the Lef-1 promoter for induction by Wnt signaling [Liu et al., 2010; Xie et al., 2014]. Second, Wnt induction raises the level of transcriptionally active β-catenin in glandular stem cells leading to the transcriptional activation of the Lef-1 promoter through the binding of TCF/β-catenin complexes [Liu et al., 2010; Xie et al., 2014]. The figure shows a schematic of early stage I PGP formation and corresponding phenotypic expression of Lef-1 (red), Sox2 (green), and Wnt activation (blue) by immunofluorescence. Wnt activation is indexed by a transgenic reporter line that express an EGFP-histone-28 fusion protein under the direction of a TCF-responsive promoter [Ferrer-Vaquer et al., 2010].