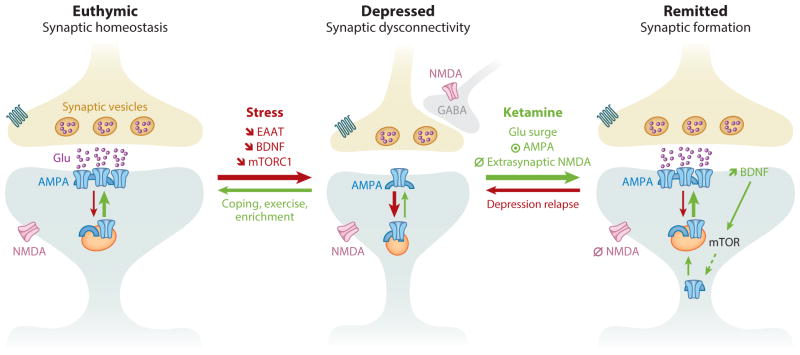
Figure 2.
Prefrontal synaptic connectivity during normal mood, depression, and after remission of depression. In euthymic individuals, stimulus and circuit activities maintain and regulate synaptic strength. Following prolonged stress and depression, an overall synaptic dysconnectivity is observed along with significant reduction in glutamate neurotransmission, excitatory amino acids transporters (EAATs), BDNF expression and release, and mTORC1 signaling. Ketamine’s rapid restoration of prefrontal synaptic connectivity is believed to result from the following consecutive events. (1) Blockade of NMDA receptors located on inhibitory GABAergic interneurons, leading to a stimulus-independent widespread prefrontal glutamate surge. (2) Activation of AMPA receptors combined with blockade of extrasynaptic NMDA receptors. (3) Increased BDNF release and activation of mTORC1 signaling, which in turn increases protein synthesis and AMPA cycling. Abbreviations: ⦿, activate; ∅, block;
 , decrease; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BDNF, brain-derived neurotrophic factor; GABA, γ-aminobutyric acid; mTORC1, mammalian target of rapamycin complex 1.
, decrease; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BDNF, brain-derived neurotrophic factor; GABA, γ-aminobutyric acid; mTORC1, mammalian target of rapamycin complex 1.