Figure 6.
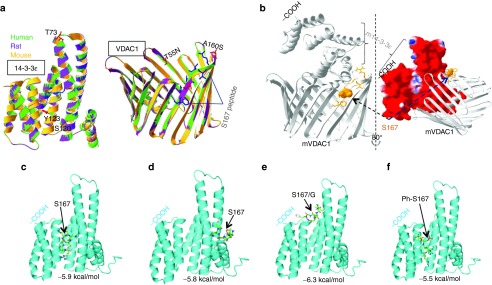
Modeling the human voltage-dependent anion channel (VDAC1) 14-3-3ɛ motif containing S167. (a) Putative models 14-3-3ɛ and VDAC1 were mapped in indicated species showing a high degree of homology. (b) Macromolecular docking among two proteins. The docking site of 14-3-3ɛ in the VDAC1 structure in Mus musculus was predicted. TVS167 was shown to dock onto open and non-ligand-bound 14-3-3ɛ at the site to which VDAC1 also bound to this protein, suggesting that TV167 can block this interaction. Due to a high percentage of homology between the 3-D structures of human 14-3-3ɛ and VDAC1, the same docking sites were predicted in these species. The ribbon representative of each protein and surface mapping of electrostatic potential of mouse 14-3-3ɛ are shown. The red, blue, and white on the molecular surface are corresponding to negative, positive, and neutral charged regions, respectively. (c,d) The molecular docking studies show TVS167 targets the 14-3-3ɛ binding groove as well as the right shoulder that interacts with VDAC1(6b). (e) Mutation of S167 to G167 removes the ability of the TV peptide to dock outside the binding groove. (f) The phosphorylated TVS167 docked within the binding groove.
