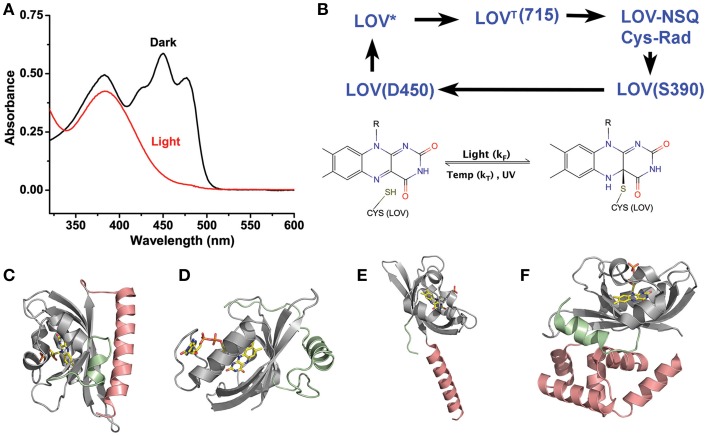
Figure 1.
LOV chemistry and structure. (A) Typical photocycle spectra of LOV containing proteins. Dark state proteins (black) demonstrate spectra consistent with oxidized flavin. Light activation (red) bleaches the 450 nm absorbing bands leaving a single 390 nm peak indicative of a C4a adduct. (B) LOV photocycles are characterized by a ground state oxidize flavin that form a flavin-cysteine C4a adduct following blue-light treatment. Adduct formation proceeds through an excited singlet state (LOV*) that rapidly forms a Triplet species (LOVT). The triplet abstracts an electron from C38 generating a radical pair. Radical recombination forms the C4a adduct (LOV390). The adduct decays to the ground state by either thermal decay (kT) or UV-scission. (C–F) Structures of LOV proteins involved in optogenetic tools, AsLOV2 (C), VVD (D), YtvA (E), and EL222 (F). The LOV core is depicted in gray, with associated N-terminal caps (green) and C-terminal caps (salmon).