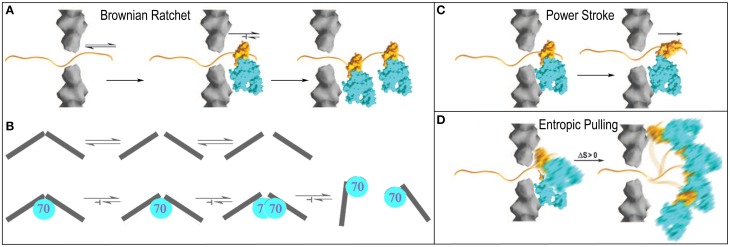
Figure 4.
Proposed mechanisms of Hsp70 mediated chemical to mechanical energy transformations (Sousa and Lafer, 2006). (A) Brownian ratchet in the context of protein translocation. An unfolded protein can slide randomly back-and-forth through a translocation pore, but Hsp70s on one side can bind the protein so that it is trapped and eventually fully translocated to one side. (B) The ratchet in the context of clathrin coat disassembly. Fluctuations that loosen interactions in the coat occur spontaneously but when Hsp70 binds it blocks reversal of these fluctuations to the ground state. Accumulation of fluctuations causes disassembly (Xing et al., 2010). (C) Power-stroke. An Hsp70 binds to a protein emerging from a translocation pore and undergoes an ATP-hydrolysis coupled conformational change that pulls the protein through the pore. (D) Entropic pulling (De los Rios et al., 2006; Goloubinoff and De Los Rios, 2007). Binding of an Hsp70 to a polypeptide segment emerging from a translocation pore restricts the freedom of motion of the Hsp70. Movement of the Hsp70 away from the pore leads to greater freedom of motion and entropy, and to a favorable free energy change. Mechanisms are presented in the context of protein translocation but are easily extended to reactions in which Hsp70 pulls proteins out of aggregates.