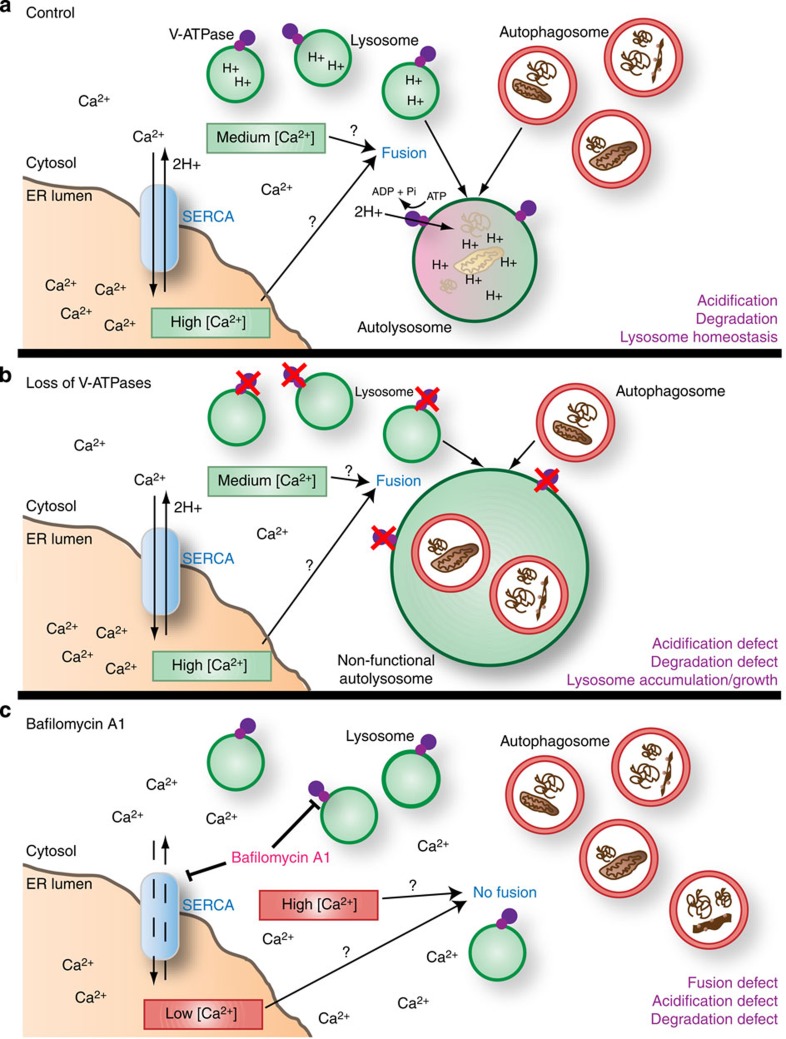
Figure 7. Schematic model of fusion and acidification events.
(a) Under normal conditions, SERCA generates a Ca2+ gradient with a high concentration of Ca2+ in the ER lumen. The role of SERCA in autophagosome–lysosome fusion may reflect its ability to maintain a relatively low/moderate cytoplasmic Ca2+ concentration, or to allow localized release of ER lumenal Ca2+ near sites of fusion. Pumping of protons into the lysosomal lumen by the V-ATPase leads to acidification, activation of hydrolytic enzymes and autophagic cargo degradation. The V-ATPase, along with amino acids released by autophagic breakdown, also promotes activation of mTOR (not shown), which is required for lysosomal reformation. (b) Genetic depletion of V-ATPase subunits results in a loss of lysosomal acidification, while Ca2+/SERCA-dependent fusion remains active. Continued autophagosome–lysosome fusion in the absence of lysosomal degradation and recycling disrupts lysosomal homeostasis, leading to an expansion of the autolysosomal compartment. (c) Inhibition of both the V-ATPase and SERCA by BafA1 blocks lysosomal acidification and dissipates the ER Ca2+ gradient. Either abnormally high Ca2+ concentration in the cytosol and/or lower Ca2+ concentration in the ER lumen may contribute to defective autophagosome–lysosome fusion.