Abstract
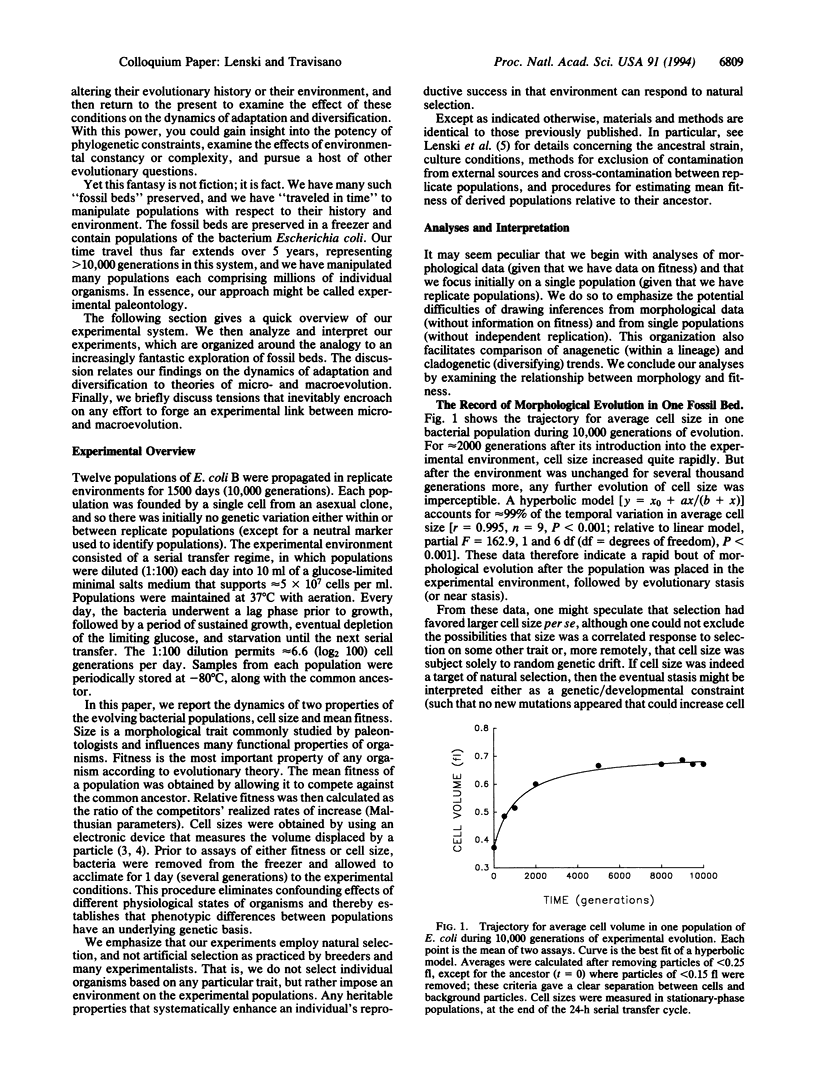
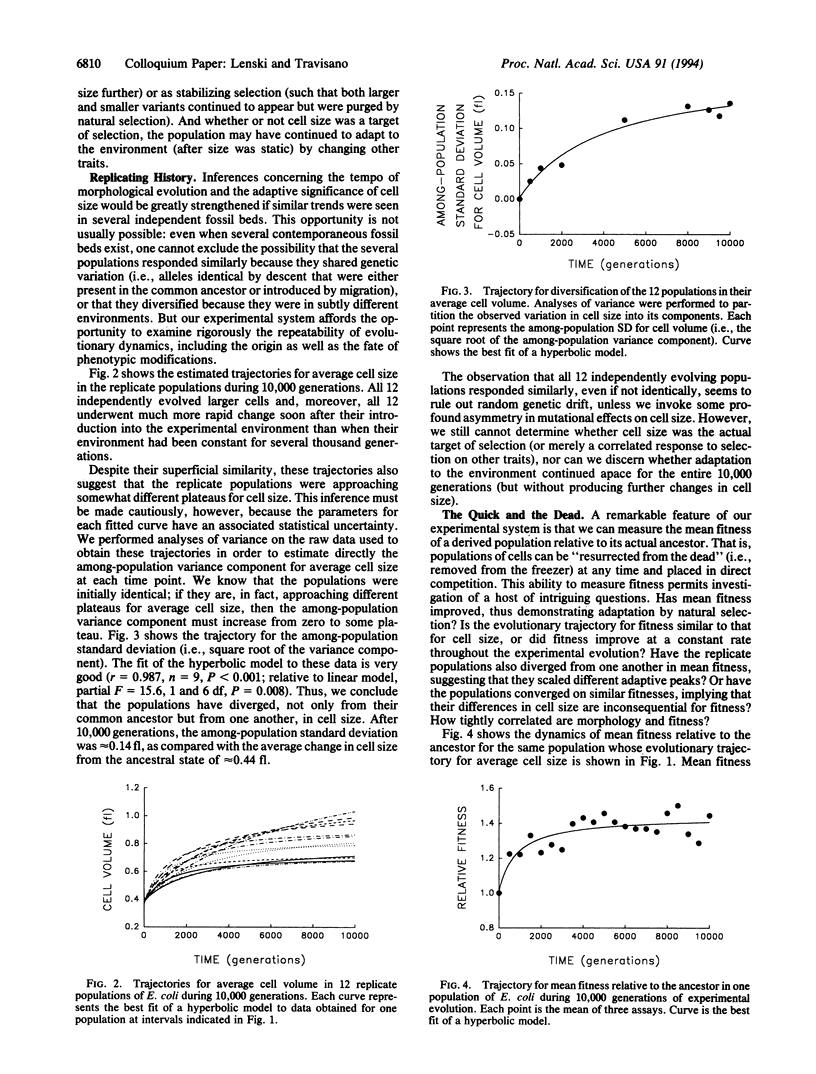
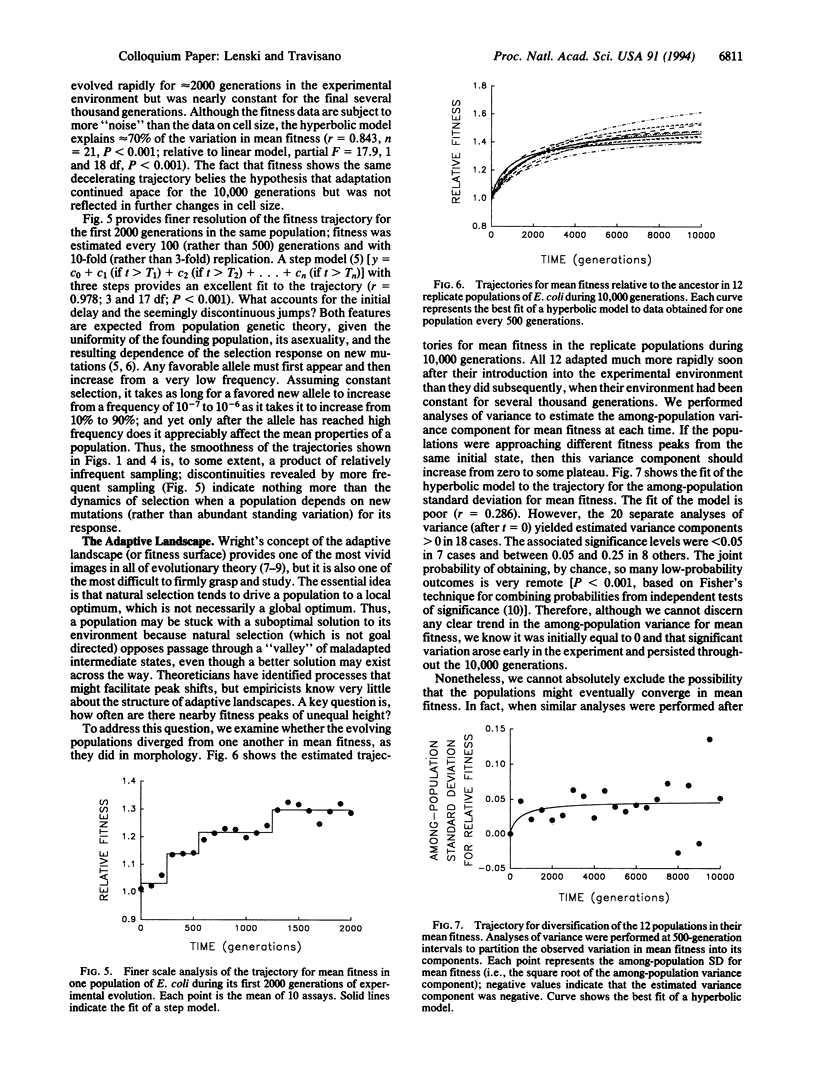
We followed evolutionary change in 12 populations of Escherichia coli propagated for 10,000 generations in identical environments. Both morphology (cell size) and fitness (measured in competition with the ancestor) evolved rapidly for the first 2000 generations or so after the populations were introduced into the experimental environment, but both were nearly static for the last 5000 generations. Although evolving in identical environments, the replicate populations diverged significantly from one another in both morphology and mean fitness. The divergence in mean fitness was sustained and implies that the populations have approached different fitness peaks of unequal height in the adaptive landscape. Although the experimental time scale and environment were microevolutionary in scope, our experiments were designed to address questions concerning the origin as well as the fate of genetic and phenotypic novelties, the repeatability of adaptation, the diversification of lineages, and thus the causes and consequences of the uniqueness of evolutionary history. In fact, we observed several hallmarks of macroevolutionary dynamics, including periods of rapid evolution and stasis, altered functional relationships between traits, and concordance of anagenetic and cladogenetic trends. Our results support a Wrightian interpretation, in which chance events (mutation and drift) play an important role in adaptive evolution, as do the complex genetic interactions that underlie the structure of organisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton N. H., Hewitt G. M. Adaptation, speciation and hybrid zones. Nature. 1989 Oct 12;341(6242):497–503. doi: 10.1038/341497a0. [DOI] [PubMed] [Google Scholar]
- Barton N. H., Rouhani S. The frequency of shifts between alternative equilibria. J Theor Biol. 1987 Apr 21;125(4):397–418. doi: 10.1016/s0022-5193(87)80210-2. [DOI] [PubMed] [Google Scholar]
- Drake J. W. A constant rate of spontaneous mutation in DNA-based microbes. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7160–7164. doi: 10.1073/pnas.88.16.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Eldredge N. Punctuated equilibrium comes of age. Nature. 1993 Nov 18;366(6452):223–227. doi: 10.1038/366223a0. [DOI] [PubMed] [Google Scholar]
- Lande R. The maintenance of genetic variability by mutation in a polygenic character with linked loci. Genet Res. 1975 Dec;26(3):221–235. doi: 10.1017/s0016672300016037. [DOI] [PubMed] [Google Scholar]
- Mani G. S., Clarke B. C. Mutational order: a major stochastic process in evolution. Proc R Soc Lond B Biol Sci. 1990 May 22;240(1297):29–37. doi: 10.1098/rspb.1990.0025. [DOI] [PubMed] [Google Scholar]
- Templeton A. R. The theory of speciation via the founder principle. Genetics. 1980 Apr;94(4):1011–1038. doi: 10.1093/genetics/94.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M. J., Goodnight C. J. Wright's shifting balance theory: an experimental study. Science. 1991 Aug 30;253(5023):1015–1018. doi: 10.1126/science.1887214. [DOI] [PubMed] [Google Scholar]


