Abstract
Introduction
Current clinical classifications of chronic rhinosinusitis (CRS) have been largely defined based upon preconceived notions of factors thought to be important, such as polyp or eosinophil status. Unfortunately, these classification systems have little correlation with symptom severity or treatment outcomes. Unsupervised clustering can be used to identify phenotypic subgroups of CRS patients, describe clinical differences in these clusters and define simple algorithms for classification.
Methods
A multi-institutional, prospective study of 382 patients with CRS who had failed initial medical therapy completed the SinoNasal Outcome Test (SNOT-22), Rhinosinusitis Disability Index (RSDI), Short Form-12 (SF-12), Pittsburgh Sleep Quality Index (PSQI), and Patient Health Questionnaire (PHQ-2). Objective measures of CRS severity included Brief Smell Identification Test (B-SIT), CT and endoscopy scoring. All variables were reduced and unsupervised hierarchical clustering was performed. After clusters were defined, variations in medication usage were analyzed. Discriminant analysis was performed to develop a simplified, clinically useful algorithm for clustering.
Results
Clustering was largely determined by age, severity of patient reported outcome measures, depression and fibromyalgia. CT and endoscopy varied somewhat among clusters. Traditional clinical measures including polyp/atopic status, prior surgery, B-SIT and asthma did not vary among clusters. A simplified algorithm based upon productivity loss, SNOT-22 score and age predicted clustering with 89% accuracy. Medication usage among clusters did vary significantly.
Discussion
A simplified algorithm based upon hierarchical clustering is able to classify CRS patients and predict medication usage. Further studies are warranted to determine if such clustering predicts treatment outcomes.
Keywords: phenotype, sinusitis, cluster analysis, quality of life, staging
Introduction
Chronic rhinosinusitis (CRS) is a heterogeneous disease currently defined by at least 3 months of cardinal sinonasal symptoms, along with visible evidence of inflammation on either physical exam or imaging study.1 Although this definition is useful to differentiate CRS from other upper airway conditions with similar symptoms, these diagnostic criteria are notably broad in nature. It has long been suggested that CRS is not a single entity, but instead many subtle clinical phenotypes are likely to exist with different underlying pathophysiologies. Consensus guidelines have recommended clinical classification of CRS into those with polyps (CRSwNP) and those without polyps (CRSsNP).2 Clinicians who treat CRS implicitly “phenotype” patients during the course of day to day practice. In addition to polyp status, this involves other demographic and medical comorbidities thought to be influential, such as age, gender and presence of asthma or atopy. Attempts to further refine the classification of CRS patients have also been made by quantifying the eosinophilic inflammation of sinonasal tissue.3,4
Unfortunately all of the current classifications remain quite broad and fail to account for the spectrum of inflammation and clinical presentation. While nasal polyps in the United States and Europe are predominantly eosinophilic, there are significant numbers of CRSwNP patients with varying degrees of neutrophilic inflammation, and conversely there are numerous CRSsNP patients who have eosinophilic inflammation but have not yet developed polyps that can be detected endoscopically.5,6 Researchers have examined symptom severity and treatment outcomes in patients based upon these different classification systems, some simply based upon polyp or eosinophil status and others more complex, utilizing many clinical measures. In most of these studies, noteworthy clinical measures (ie computed tomography (CT) scores, asthma, atopy, eosinophil status) often fail to predict clinical outcomes, calling into question their utility.7–10
Identification of CRS subgroups is critical on multiple levels. From a clinical standpoint, appropriately classifying a patient with CRS might allow for better disease prognostication and help to guide patient decision-making with regards to medical or surgical treatments. Subclassification of CRS is also critical to research efforts aimed at understanding endotypes of CRS. Endotypes are subclasses of disease that share a common underlying pathophysiologic mechanism. If patients are enrolled into research protocols using only broad classifications, then inherent heterogeneity can reduce power and confuse findings, particularly in biomolecular studies where sample sizes are often small. Further refining clinical phenotypes thus complements studies aimed at understanding underlying endotypes and developing targeted therapeutics.
A major limitation of our current CRS classifications is that subgroups are typically defined a priori based upon the researcher’s preconceived notion of what is or is not important. Asthma researchers deal with a similar heterogeneous disorder and unsupervised clustering has been used to develop less biased clinical classifications of asthma.11 The aim of this study was to 1) utilize unsupervised clustering methods to identify phenotypic subgroups from a large, multi-institutional prospective cohort of patients with CRS, 2) describe clinical differences in identified clusters, and 3) perform discriminant analysis in order define a simple algorithm to classify patients into identified clusters.
Methods
Study cohort
Study participants were prospectively recruited from rhinologic practices at 4 tertiary medical centers across North America (Medical University of South Carolina, Stanford University, University of Calgary, and Oregon Health and Sciences University). All patients had CRS as defined by consensus criteria, including 3 months of at least 2 cardinal symptoms and evidence of inflammation on sinonasal endoscopy and CT scan.1,12 All patients had ongoing symptoms after initial attempts at medical treatment, including broad spectrum or culture-directed antibiotics, oral steroids, and topical steroids. Importantly, this cohort thus does not include those patients with CRS whose symptoms resolve with initial medical therapy. We also excluded patients with cystic fibrosis, ciliary dyskinesia or other autoimmune disorders, as these are discrete disorders with unique pathophysiologies. Fungus ball or mucoceles were also excluded since they are well identified clinical entities with straightforward treatment outcomes.
After informed consent was obtained, research coordinators administered questionnaires that assessed demographic information, medical comorbidities, medication usage over the preceding 90 days, and productivity loss (work days missed in the preceding 90 days). Sinus-specific quality of life (QOL) was assessed using the 22-item Sinonasal Outcome Test (SNOT-22) and Rhinosinusitis Disability Index (RSDI) instruments.13,14 General QOL was assessed using the Medical Outcomes Study Short Form-12 (SF-12).15 Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI).16 Depressed mood and anhedonia were assessed using the Patient Health Questionnaire-2 (PHQ-2).17 Objective olfactory function was evaluated using the Brief Smell Identification Test (B-SIT).18 For each subject, CT scans were reviewed in blinded fashion and graded according to Lund-Mackay staging system.19 Sinonasal endoscopy was performed and grading according to the Lund-Kennedy system, with reviewers blinded to patient-reported clinical data.20
Variable reduction
The dataset consisted of a total of 103 variables. This included 3 demographic variables, 14 comorbidity/exposure variables, 3 objective measures of CRS and 83 patient-reported outcome measure (PROM) variables, including those from five validated questionnaires. Prior to performing a cluster analysis, PROM variables were reduced to meaningful factors which contained questions with a high degree of correlation. Corresponding composite scores were determined. For instance, the SNOT-22 questionnaire, which consisted of 22 individual questions related to sinonasal QOL, was reduced to three factors. Similarly, the RSDI was reduced to four factors and the SF-12 to two factors (Table 1). The PSQI and PHQ did not reduce to meaningful factors, thus total scores were used. After the factor analysis of the PROMs, the 103 original variables were reduced to 32 variables (Table 2). These variables encompass the range of typical clinical assessment of patients with CRS, including but not limited to demographic data (age, gender, race/ethnicity), medical comorbidities thought to influence CRS (atopy, asthma, diabetes), prior sinus surgery, exposures (smoke, alcohol), objective CRS severity metrics (CT, endoscopy, olfaction), and patient-reported outcome measures (QOL, sleep quality, depression, productivity loss). Similar to cluster analyses performed in asthma, we defined “clinical phenotype” to be a visible characteristic of an organism that results from the interaction between the genetic makeup and the environment.21 Thus PROMs were considered to be a result of this interaction and a clinically observable variable, similar to prior cross sectional cluster analyses performed in asthma.11 Subjects were required to have all 32 variables to be included in the cluster analysis. One patient was excluded after being identified as an outlier; their alcohol consumption was more than 3.5 standard deviations greater than the second greatest value and more than 11 standard deviations greater than the mean. Unlike asthma, well defined algorithms for use of medications does not exist for CRS, but varies widely among practitioners based upon preconceived notions of what is effective, often with little supportive evidence. Thus, medication usage by class was not used to determine clusters, as it was not a specific characteristic of a given patient’s CRS, but rather a factor determined by the treating physician. After the 32 variables above were used to define clusters, recorded variations in medication usage were analyzed.
TABLE 1.
Variable reduction in PROMS
Sinonasal Outcomes Test 22 (SNOT-22)
|
Rhinosinusitis Disability Index (RSDI)
|
12-Item Short Form Health Survey (SF-12)
|
Factor analysis was done in order to identify correlated variables, allowing reduction into a smaller number of factors to be utilized in the cluster analysis.
Table 2.
Variables Used to Define Clusters
| Variable | Total N = 382 |
Cluster 1 N = 49 (13%) |
Cluster 2 N = 75 (19%) |
Cluster 3 N = 166 (43%) |
Cluster 4 N = 61 (16%) |
Cluster 5 N = 31 (8%) |
p |
|---|---|---|---|---|---|---|---|
| DEMOGRAPHICS | |||||||
| Gender | |||||||
| Female | 189 (49.5%) | 18 (36.7%) | 31 (41.3%) | 93 (56.0%) | 26 (42.6%) | 21 (67.7%) | 0.010 |
| Male | 193 (50.5%) | 31 (63.3%) | 44 (58.7%) | 73 (44.0%) | 35 (57.4%) | 10 (32.3%) | |
| Race | |||||||
| African American | 23 (6.0%) | 3 (6.1%) | 4 (5.3%) | 9 (5.4%) | 5 (8.2%) | 2 (6.5%) | 0.045 |
| White | 317 (83.0%) | 42 (85.7%) | 66 (88.0%) | 138 (83.1%) | 42 (68.9%) | 29 (93.5%) | |
| Other | 42 (11.0%) | 4 (8.2%) | 5 (6.7%) | 19 (11.4%) | 14 (23.0%) | 0 (0.0%) | |
| Age (years) | 49.9 (14.7) | 65.71 (7.8) | 63.3 (7.9) | 42.6 (11.9) | 39.3 (9.4) | 53.0 (12.7) | <0.001 |
| COMORBIDITIES/EXPOSURES | |||||||
| Diabetes | 20 (5.2%) | 7 (14.3%) | 4 (5.3%) | 4 (2.4%) | 1 (1.6%) | 4 (12.9%) | 0.003 |
| AFRS | 10 (2.6%) | 0 (0.0%) | 0 (0.0%) | 7 (4.2%) | 3 (4.9%) | 0 (0.0%) | 0.131 |
| Allergy History | 50 (13.1%) | 10 (20.4%) | 13 (17.3%) | 15 (9.0%) | 7 (11.5%) | 5 (16.1%) | 0.179 |
| Prior Sinus Surgery | 153 (40.0%) | 18 (36.7%) | 31 (41.3%) | 71 (42.8%) | 20 (32.8%) | 13 (41.9%) | 0.704 |
| Asthma | 131 (34.3%) | 12 (24.5%) | 24 (32.0%) | 69 (41.6%) | 16 (26.2%) | 10 (32.3%) | 0.092 |
| Polyps | 130 (34.0%) | 18 (36.7%) | 27 (36.0%) | 60 (36.1%) | 19 (31.1%) | 6 (19.4%) | 0.431 |
| ASA Intolerance | 31 (8.1%) | 4 (8.2%) | 5 (6.7%) | 17 (10.2%) | 4 (6.6%) | 1 (3.2%) | 0.661 |
| OSA | 49 (12.8%) | 7 (14.3%) | 10 (13.3%) | 23 (13.9%) | 5 (8.2%) | 4 (12.9%) | 0.838 |
| COPD | 12 (3.1%) | 2 (4.1%) | 3 (4.0%) | 5 (3.0%) | 1 (1.6%) | 1 (3.2%) | 0.940 |
| Depression | 64 (16.8%) | 1 (2.0%) | 10 (13.3%) | 31 (18.7%) | 7 (11.5%) | 15 (48.4%) | <0.001 |
| Fibromyalgia | 17 (4.5%) | 0 (0.0%) | 4 (5.3%) | 8 (4.8%) | 0 (0.0%) | 5 (16.1%) | 0.004 |
| Oral Steroid Dependence | 21 (5.5%) | 3 (6.1%) | 5 (6.7%) | 9 (5.4%) | 3 (4.9%) | 1 (3.2%) | 0.965 |
| Alcohol (grams/week) | 28.4 (51.6) | 44.72 (63.4) | 39.1 (67.3) | 24.5 (45.8) | 18.4 (26.4) | 16.3 (46.8) | 0.011 |
| Smoker (pack/day) | 0.02 (0.14) | 0 (0) | 0.01 (0.04) | 0.04 (0.2) | 0.01 (0.06) | 0.03 (0.12) | 0.366 |
| OBJECTIVE CRS SEVERITY MEASURES | |||||||
| Endoscopy Total | 5.9 (3.8) | 5.9 (3.9) | 5.9 (4.0) | 6.4 (3.9) | 4.4 (3.3) | 6.3 (3.4) | 0.014 |
| CT Total | 11.6 (6.2) | 11.51 (6.4) | 13.1 (6.1) | 11.7 (6.1) | 9.0 (6.0) | 12.7 (5.7) | 0.002 |
| B-SIT Total | 9.0 (3.1) | 9.02 (3.1) | 8.6 (3.4) | 9.0 (3.2) | 10.0 (2.7) | 8.9 (2.7) | 0.186 |
| PATIENT REPORTED OUTCOME MEASURES | |||||||
| SNOT-22 Total | 51.1 (20.4) | 22.57 (9.9) | 47.7 (8.1) | 64.1 (13.7) | 31.0 (9.0) | 74.0 (11.4) | <0.001 |
| SNOT factor 1 (fatigue) | 21.5 (11.7) | 6.7 (6.0) | 20.0 (7.3) | 28.3 (7.9) | 10.2 (7.9) | 34.1 (5.8) | <0.001 |
| SNOT factor 2 (drainage/emotional) | 21.1 (7.6) | 13.8 (8.0) | 20.8 (5.9) | 24.4 (6.2) | 15.9 (6.3) | 26.7 (6.0) | <0.001 |
| SNOT factor 3 (ear/smell/congestion) | 8.5 (5.5) | 2.2 (2.0) | 6.9 (4.1) | 11.5 (4.8) | 4.9 (2.6) | 13.3 (4.9) | <0.001 |
| RSDI Total | 44.6 (24.1) | 17.3 (12.5) | 37.9 (17.4) | 55. 8 (18.7) | 27.9 (15.8) | 77.3 (17.1) | <0.001 |
| RSDI factor 1 (emotion) | 12.3 (8.8) | 4.6 (5.2) | 10.0 (7.0) | 15.5 (8.1) | 7.2 (6.4) | 22.6 (7.8) | <0.001 |
| RSDI factor 2 (physical) | 15.6 (9.4) | 5.6 (5.5) | 13.1 (7.3) | 19.3 (7.5) | 9.9 (6.6) | 28.4 (7.1) | <0.001 |
| RSDI factor 3 (phys/pain/exertion) | 9.1 (5.7) | 3.2 (3.3) | 7.4 (4.5) | 11.5 (4.7) | 6.1 (4.4) | 15.8 (4.8) | <0.001 |
| RSDI factor 4 (phys/drain/olfaction) | 7.7 (3.9) | 3.9 (3.1) | 7.4 (3.0) | 9.5 (3.2) | 4. 7 (3.0) | 10.5 (3.3) | <0.001 |
| PSQI Total | 9.1 (4.6) | 4.98 (2.7) | 8.6 (3.7) | 10.7 (4.3) | 6.1 (3.1) | 13.7 (4.5) | <0.001 |
| PHQ Total | 1.5 (1.6) | 0.5 (1.2) | 1.3 (1.4) | 1.9 (1.6) | 1.0 (1.3) | 3.2 (2.0) | <0.001 |
| SF 12 total | 31.5 (2.7) | 31.2 (2.3) | 31.1 (2.2) | 31.8 (3.0) | 32.0 (2.5) | 30.7 (2.7) | 0.039 |
| SF 12 factor 1 | 16.6 (2.0) | 15.0 (1.8) | 16.2 (1.7) | 17.2 (2.1) | 16.6 (2.0) | 17.4 (1.4) | <0.001 |
| SF 12 factor 2 | 14.9 (2.1) | 16.2 (1.6) | 14.9 (2.0) | 14.6 (2.1) | 15.4 (1.8) | 13.2 (2.4) | <0.001 |
| Productivity loss | 8.6 (18.1) | 1.14 (3.6) | 3.1 (5.5) | 6.0 (8.3) | 2.3 (4.2) | 60.7 (24.0) | <0.001 |
Variables used to define clusters are presented as either count data and percentages or means and standard deviations. AFRS=allergic fungal rhinosinusitis; CT=computed tomography; B-SIT=Brief Smell Identification Test; SNOT-22=Sinonasal Outcomes Test 22; RSDI=Rhinosinusitis Disability Index; PSQI=Pittsburgh Sleep Quality Index; PHQ= Patient Health Questionnaire-2; SF12=Medical Outcomes Study Short Form-12; productivity loss=missed days of work out of last 90 days.
Statistical analysis
All analyses were performed using SAS 9.4 ©. Ward’s minimum-variance hierarchical method was used to perform the cluster analysis. This analysis places subjects into groups, or clusters, suggested by the data, not defined a priori. Therefore, subjects in a given cluster tend to be similar to each other in some sense, and subjects in different clusters tend to be dissimilar. Cluster analysis can only be performed on datasets without missing variables; therefore, those subjects with missing data were removed. Chi-square tests for categorical measures and t-tests for continuous measures were performed for all variables between the complete data set used in the cluster analysis and the data set of individuals not used in the cluster analysis because of missing data in order to ensure no major differences existed. Once clusters were generated, differences between clusters for all recorded risk factors were tested using analysis of variance for continuous measures and chi-square for categorical measures.
Backward stepwise discriminant analysis was performed on all variables used in the cluster analysis to identify the main variables that distinguish the clusters. From the discriminant analysis, a set of variables capable of discriminating the study population into their respective clusters was identified. Binary classification trees were generated by defining thresholds for each of the variables in this set through clinical considerations. A sensitivity analysis was performed to examine the validity of the intuitive thresholds by shifting them over a range of +/− 5 units. A resampling algorithm was used to determine the distribution of the estimate of classification error in all combinations of cut-off points that performed equally best in the binary classification tree analysis. The resampling was performed 1000 times for each combination of cut-off values. Statistical significance was assessed at α = 0.05. When testing differences between clusters and sample subsets, statistical significance was adjusted using the Bonferroni correction.
Results
Study cohort and demographics
The overall dataset included 539 patients ranging in age from 18–86, of whom 482 patients remained after exclusion criteria were applied. Of these, 382 patients who had complete data constituted the analysis data set. No significant difference was found between the 382 patients with complete data and 100 patients with incomplete data across all variables (data not shown). The demographics and clinical characteristics for the final cohort are presented in the first column of Table 2. The total cohort was equally split between genders and 34% (130/382) had nasal polyps. Overall, 40% (153/382) of patients had undergone prior sinus surgery and comorbidities such as atopy (13%, 49/382) and asthma (34%, 131/382) were common.
Cluster Analysis
Using the clustering approach outlined above, a dendrogram was generated as shown in Figure 1. A five-cluster reduction was chosen to describe outcomes. Differences across clusters are presented for demographic factors and medical comorbidities, disease severity metrics, PROM measures, and medication usage (Tables 2 and 3). Interestingly, traditional clinical characteristics such as presence of polyps, atopy, asthma, aspirin sensitivity, allergic fungal rhinosinusitis, and history of prior sinus surgery did not differ to any significant degree across clusters. Objective measures of CRS severity, such as CT and endoscopy, did vary somewhat as described below, however, B-SIT scores did not vary among clusters.
Figure 1.
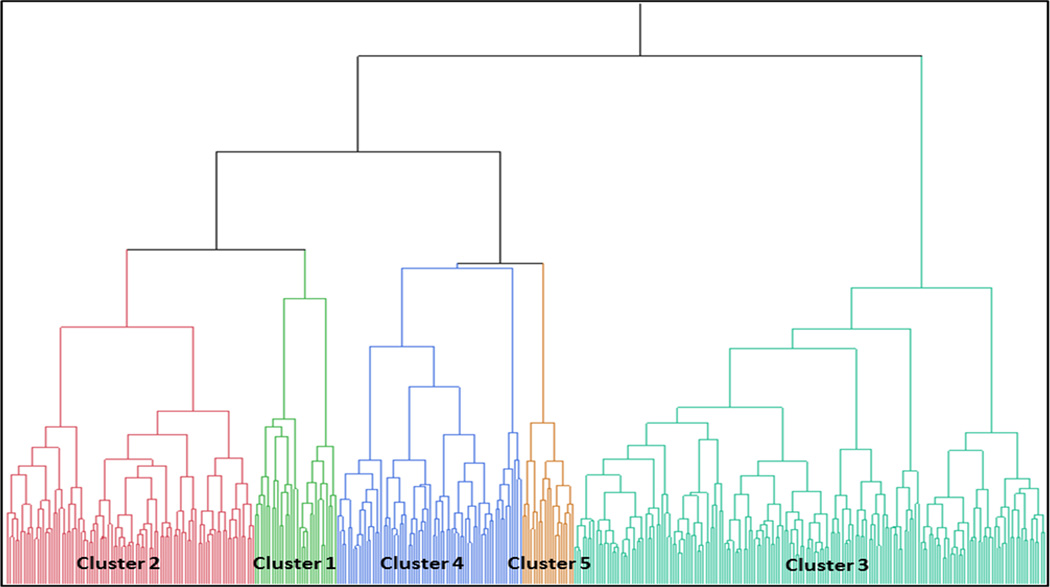
Dendrogram for development of 5 clusters
Table 3.
Medication usage by Cluster
| Medication Usage in Last 90 Days by Cluster | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Total N = 382 |
Cluster 1 N = 49 |
Cluster 2 N = 75 |
Cluster 3 N = 166 |
Cluster 4 N = 61 |
Cluster 5 N = 31 |
p |
| Steroid Drops | 9.94 (26) | 13.72 (31.54) | 5.79 (19.57) | 9.97 (23.97) | 19.84 (35.88) | 0.031 | |
| Steroid nasal spray | 44.67 (39.23) | 49.12 (38.56) | 38.36 (38.18) | 36.38 (37.93) | 52.42 (39.1) | 0.101 <.001 |
|
| Decongestants | 10.84 (25.08) | 16.64 (26.91) | 25.06 (34.01) | 11.57 (24.18) | 36.68 (41.6) | ||
| Oral Antibiotics | 13.43 (20.11) | 15.36 (21.18) | 15.55 (19.85) | 12.47 (18.21) | 31.81 (30.5) | <.001 | |
| Oral Steroids | 13.06 (22.94) | 11.6 (19.86) | 11.59 (18.62) | 8.32 (15.79) | 20.77 (29.13) | 0.089 | |
| Oral Antihistamines | 16.34 (30.81) | 25.77 (35.97) | 27.31 (36.6) | 16.98 (31.54) | 30.13 (40.14) | 0.13 | |
| Leukotriene Antagonists | 19.63 (35.29) | 17.46 (34.53) | 16.14 (31.97) | 8.15 (22.21) | 18.71 (35.66) | 0.33 | |
| Saline spray/irrigations | 30.18 (34.47) | 47.69 (38.41) | 39.15 (36.67) | 42.85 (37.85) | 57.23 (35.31) | 0.012 | |
Cluster 1
Thirteen percent of patients are grouped into Cluster 1. This cluster is characterized by the highest percentage of males (63%) and is the oldest cluster (mean age of 65 years). Cluster 1 has a higher frequency of type 2 diabetes (14%) and more alcohol intake (3.1 drinks/week). This cluster had the lowest rate of depression (2%) compared to other clusters. Objective disease severity measures were intermediate, with a mean CT score of 11.5 (SD = 6.4) and endoscopy score of 5.9 (SD = 3.9). Despite intermediate objective disease measures, this group reported the least severe PROMs, including sinus-specific QOL, general QOL, sleep quality and productivity loss.
Cluster 2
Cluster 2 contains 19% of patients and also has slightly more men (59%) than women and tends to have older patients (mean of 63 years). Patients in this cluster were more likely to report depression (13%) as compared to Cluster 1 (2%). Despite the most severe CT scores (mean=13.1; SD=6.1) and severe endoscopy scores (mean = 5.9, SD = 4.0), their sinus-specific QOL, general QOL, sleep quality and productivity loss were intermediate in severity.
Cluster 3
Cluster 3 is the largest cluster (n=166; 43%) and has slightly more women (56%) than men. From a comorbidity standpoint, depression was more common in this cluster (19%). Objectively, this group had the worst endoscopy scores of all the clusters (mean = 6.4, SD = 3.9), but intermediate CT scores (mean = 11.7, SD = 6.0). Except for Cluster 5 (discussed below), patients in Cluster 3 reported the worst sinus-specific QOL, general QOL, sleep quality and productivity loss.
Cluster 4
Sixteen percent of patients are grouped into cluster 4, with 57% males and the youngest mean age (39 years). Presence of medical comorbidities is similar to Clusters 2 and 3, including depression (12%). This cluster is characterized by the least severe objective disease, including CT (mean = 9.0, SD = 6.0) and endoscopy (mean = 4.4, SD = 3.3). In general, this group had worse sinus-specific QOL, general QOL, sleep quality and productivity loss compared to Cluster 1, despite less severe objective disease. However, except for Cluster 1, PROMs were less severe than the other 3 clusters.
Cluster 5
Cluster 5 is the smallest group (n=31; 8% of patients), but is characterized by the highest percentage of women (68%). Almost half (48%) of patients reported a prior diagnosis of depression and 16% reported fibromyalgia. Objectively, this cluster had worse CT (mean = 12.7, SD = 5.7) and endoscopy scores (mean = 6.3, SD = 3.4) compared to all other clusters except cluster 2. PROMs in this group displayed the most severe PHQ-2 scores, indicating depressed mood and anhedonia, as well as the worst sinus-specific QOL, general QOL, and sleep quality. Out of the last 90 days, patients in this cluster reported an average 61 days of productivity loss, with the next highest cluster reporting 6 days missed.
Medication usage
After clusters were defined, medication usage by classification was assessed. Cluster 5 used significantly more steroid drops, decongestants, antibiotics and saline than all other clusters. Medication usage among the other 4 clusters was relatively equivalent (Table 3).
Discriminant Analysis
Discriminant analysis was performed on all 32 variables used in the cluster analysis in order to identify those measures which best separate patients into clusters. Age, productivity loss, and total SNOT-22 were the most discriminating. Binary classification tree analysis was used to develop a clinical algorithm in order to place patients into appropriate clusters. The sensitivity analysis identified 12 combinations of cut-off values that performed equally well, in which patients were assigned to the appropriate clusters 89.4% of the time. The results of repeating the resampling algorithm 1,000 times per combination of cut-off values showed no difference between any combinations. Cut-off values were then chosen by what was considered most clinically meaningful. The resulting algorithm is shown in Figure 2. Appropriate classification into clusters ranged from 80% for Cluster 4 to 96% for Cluster 2 (Figure 3, Table 4). This analysis suggests that these simple clinical measures can be used to classify patients into statistical clusters with considerable accuracy.
Figure 2.
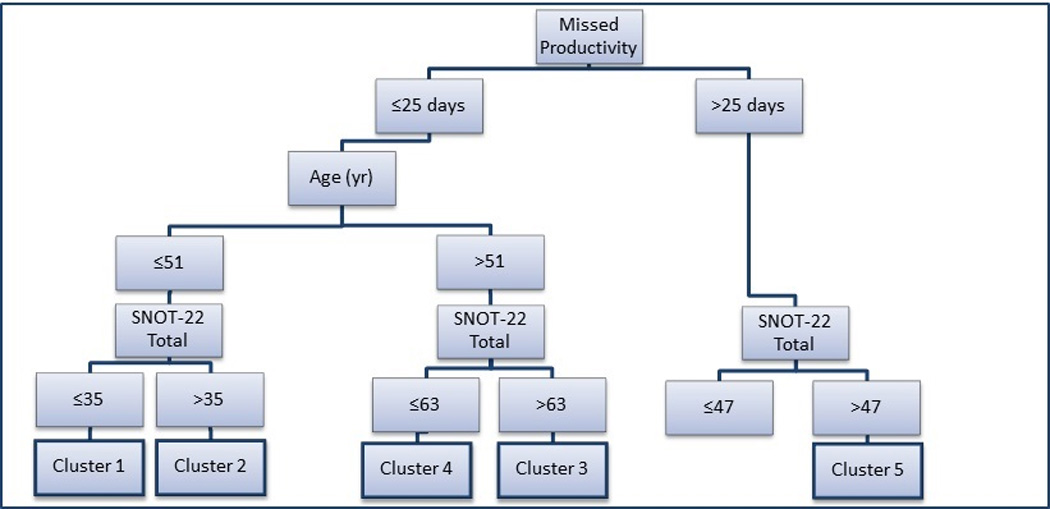
Based on the discriminant analysis, the above algorithm can be used to classify patients into the five statistical clusters using simple clinical measures. The likelihood of correct classification using this algorithm is shown in Figure 3 and Table 4. SNOT-22=22-item Sinonasal Outcome Test. Yr=years of age. Productivity loss equals the number of work days missed in last 90 days.
Figure 3.
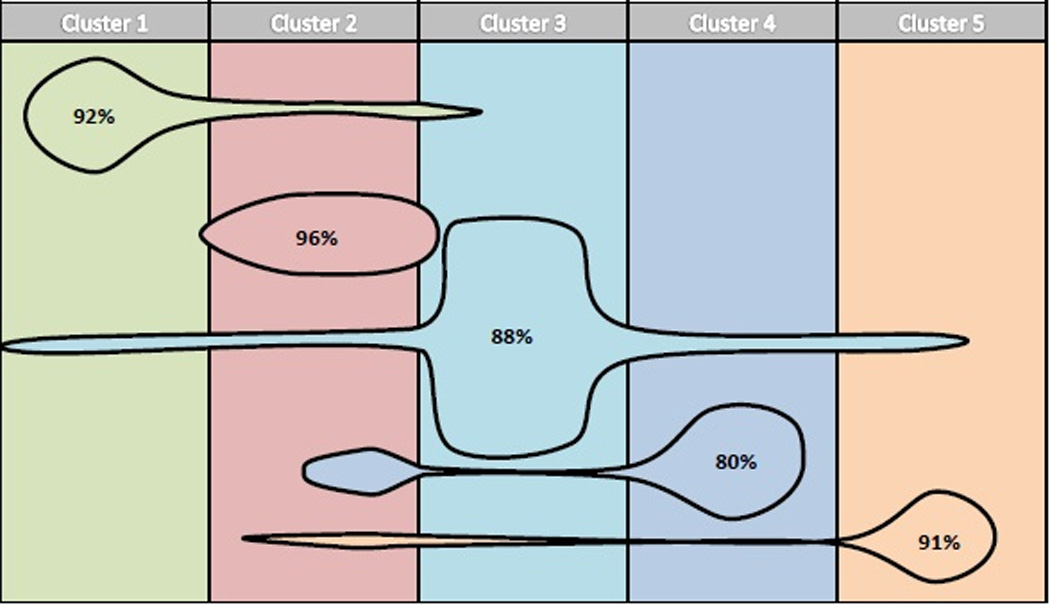
Based on the clinical algorithm developed using discriminant analysis, 89.4% of all individuals are categorized into the appropriate cluster, with individual clusters ranging from 80–96% correct classification. The size of each figure is proportional to the frequency/size of each specific cluster and overlap into other clusters signifies the percentage of misclassification.
Table 4.
Clinical algorithm performance
| Correct Cluster | Assigned Cluster | Total (%) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 1 | 45 (91.8%) | 3 (6.1%) | 1 (2.0%) | 0 (0%) | 0 (0%) | (100%) |
| 2 | 1 (1%) | 98 (96.1%) | 3 (2.9%) | 0 (0%) | 0 (0%) | (100%) |
| 3 | 5 (3.8%) | 4 (3.0%) | 116 (87.9%) | 3 (2.3%) | 4 (3.0%) | (100%) |
| 4 | 0 (0%) | 13 (18.3%) | 1 (1.4%) | 57 (80.3%) | 0 (0%) | (100%) |
| 5 | 0 (0%) | 2 (6.3%) | 1 (3.1%) | 0 (0%) | 29 (90.6%) | (100%) |
Assignment to clusters based on the clinical algorithm shown in Figure 2 was compared to the correct cluster as generated through full data analysis. For example, the clinical algorithm appropriately classified 91.8% of patients in Cluster 1 to Cluster 1, whereas 6.1% were incorrectly assigned to Cluster 2, 2.0% to Cluster 3, and none to Cluster 4 or 5.
Discussion
Current consensus groups recommend classification for CRS by polyp status, while other studies base separation upon factors such as atopy, asthma, aspirin sensitivity, and allergic fungal rhinosinusitis.1–3,7,22,23 Assessments of CRS severity have traditionally been broken down by endoscopic or CT scores, driven largely by polyp status. Unfortunately, these traditional classifications and measures of disease severity have not universally been found to impact clinically relevant features such as symptom severity, treatment selection or therapeutic response. A large prospective study investigating surgical outcomes found that the only clinical predictor of surgical success was lack of prior surgery.8 Rather than using a priori defined characteristics, the current study used unsupervised statistical methods to generate clusters based on prospectively collected clinical data from a large cohort of patients with CRS. Interestingly, traditional measures such as polyp status or atopy did not differ significantly across clusters and objective measures of CRS severity, such as CT and endoscopy only varied slightly among clusters. Instead, measures such as age, gender, productivity loss, and QOL impairment characterized differences between groups.
A challenge with statistical clustering methods is understanding the clinical relevance of the groups generated. As physicians we naturally seek to understand and organize diseases based upon clinical factors that may explain the underlying pathophysiology. This is critical because treatments often target specific mechanisms of disease. It is too preliminary to know whether the clusters generated in this analysis represent distinct pathophysiologies of CRS. Perhaps more likely, these clusters are simply describing patients which share common clinical features that may or may not share underlying mechanisms of disease. Most physicians with a busy CRS practice are likely on any given day to treat individuals who fit these clusters, such as the elderly male patient with significant disease on CT and endoscopy yet minimal symptoms/QOL impairment (Cluster 1), the person with only mild changes on CT but severe symptoms (Cluster 3), or the depressed patient who is so debilitated they can no longer work (Cluster 5). Regardless of underlying pathophysiologies, patients who fall into these various clusters result in different treatment approaches, as evidenced by the variation in medication usage seen across clusters. It is unclear if such clustering also predicts differences in treatment choices or therapeutic outcomes over time. It is important to note that our patient population represented a more severe form of CRS that had failed standard medical therapies and was deemed a potential surgical candidate. Patients responding to first line medical therapy were excluded and may represent a milder form of the disease.
Similar clustering techniques have been performed in severe asthmatics.11,21 Traditionally, these patients were classified based upon atopy, age and eosinophilia; however limited utility in predicting treatment outcomes led to unsupervised hierarchichal clustering. Interestingly, one of these studies found a cluster dominated by males with the worst objective measures of disease, but the best quality of life, similar to our cluster 1.21 Moore et. al. performed discriminant analysis of asthma clustering and defined clusters based upon age of onset and pulmonary function tests.11 In her series, discriminant analysis accurately classified asthmatic patients 80% of the time. This is in contrast to our discriminant analysis which correctly clustered all CRS patients just under 90% of the time. Future studies using discriminant analysis, rather than formal cluster analysis, will need to take into consideration that patients may not be correctly placed into certain clusters, such cluster 4, up to 20% of the time. Asthma clustering also used medication usage as a defining variable. In contrast to asthma where there are well established, evidence based guidelines for recommending medical therapies, CRS has limited evidence to support many of its therapies and this is left to the discretion of the treating physician and probably varies among specialties and even from physician to physician. We did include steroid dependence as a potential cluster defining variable, since its use would likely be fairly consistent among practitioners. However, use of other medications, such as systemic antibiotics, lack standards for choice of medication, duration and indication, thus likely do not represent inherent phenotypic characteristics and would be considered a potential confounder as described by others.21 Thus we chose to include variations in medical therapy as a descriptive variable, rather than a defining one.
Biomarkers of disease are notably absent in this dataset, as collecting these measures in a large cohort is logistically and financially challenging. Several groups, including our own, have suggested that tissue eosinophilia may impact outcomes in CRS.3,4 Biomolecular differences clearly exist on average between CRSwNP and CRSsNP, with the former displaying a more Th-2 skewed cytokine profile and the latter a mixed Th-2/Th-1 profile, however a wide spectrum of disease exists and these differences are not clear cut.24–27 Widespread differences likely exist across patients with CRS, including those seen in the genome, transcriptome, proteome, and microbiome. Including a robust set of potential biomarkers in future cluster analyses may allow further stratification of disease by these measures and an even better understanding of clinical phenotypes. An improved understanding of CRS phenotypes is a critical step towards understanding potential underlying endotypes/mechanisms of disease.
To date one other group has reported a cluster analysis of CRS patients that resulted in groups separated by mucosal eosinophilia and polyp status.28 This study by Nakayama et al differs from the current analysis in several important ways. From a demographic standpoint, subjects were enrolled from Asia only and just over 80% were of male gender, a notable contrast to the current study. The former study also excluded patients with aspirin sensitivity. Perhaps most noteworthy, validated patient-reported outcome measures were not utilized, including sinus-specific QOL, general QOL, sleep quality, or depression. When comparing these studies it is important to remember that analyses are limited to the variables utilized in the analysis, thus including/excluding certain key variables changes the clusters identified. Interestingly, if we exclude patient-reported outcome measures from our dataset and repeat the cluster analysis (data not shown) clusters separate out more by traditional measures (polyp status, asthma, CT score), similar to that seen in the Nakayama study. However, when this is done, the clusters no longer differ by clinically-relevant measures such as QOL or medication usage. As mentioned above, separating patients based upon these traditional measures has failed to correlate with symptom severity, treatment choice or therapeutic outcome.
The question remains as to whether these clusters can be utilized to improve clinical care and/or research inquiries into CRS. Using the algorithm generated in the discriminant analysis, our intention is to determine whether these clusters predict treatment choice (medical versus surgical) and therapeutic outcomes. If discrete, meaningful differences exist then these clusters might be utilized in routine clinical practice to better classify patients and improve prognostication. From a research standpoint, one could consider utilizing these clusters in hopes of generating more homogenous groups for biomolecular research. Use of these clusters would run contrary to current practice, since clusters include both CRSsNP and CRSwNP patients. Typically, CRSsNP and CRSwNP are separated, owing to average differences seen between these groups on histologic and biomolecular studies. In fact, we performed a cluster analysis using PROMs as an outcome measure, rather than a cluster-defining variable. Patients clustered into traditional groups, based largely upon polyp status, CT and endoscopy scores with little difference in PROMs across clusters, thus confirming numerous prior studies. However, it remains possible that differences between patients with CRSsNP and those with CRSwNP may be relatively subtle and have little impact upon treatment response. Larger differences in the clinical presentation and severity of CRS may be due to other factors and such classification may more strongly predict outcomes. Regardless, significantly more data is needed in order to fully stratify patients. For this reason, we aim in future studies to include an array of biomolecular markers in addition to those utilized in this study. Only with this degree of comprehensive phenotypic description are we likely to begin to understand underlying endotypes/mechanism of disease.
Conclusion
Traditional clinical phenotyping of CRS patients based upon nasal polyp status, atopy and CT/endoscopic staging has failed to correlate with clinical presentation or treatment response. Unsupervised hierarchichal clustering resulted in 5 distinct clusters that varied in medication usage. Clinical classification of CRS patients can be done using age, total SNOT22 score and productivity loss with high accuracy. It remains to be determined if such clustering will better predict treatment selection and response.
Acknowledgments
Dr. T. L. Smith, Dr. Z. M. Soler, and J. C. Mace are supported by a grant from the National Institutes of Health (NIH; R01 DC005805). The NIH had no role in the preparation, review, or approval of this article or decision to submit it for publication. Dr. Z. M. Soler and J. C. Mace received grant support from the NIH/NIDCD. Dr. R. J. Schlosser is consultant for BrainLAB, Olympus, and Arrinex; and received grant support from Intersect ENT and Optinose, NeilMed. Dr. T. L. Smith is consultant for Intersect ENT.
Footnotes
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1.Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. The Journal of allergy and clinical immunology. 2004;114:155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kountakis SE, Arango P, Bradley D, Wade ZK, Borish L. Molecular and cellular staging for the severity of chronic rhinosinusitis. The Laryngoscope. 2004;114:1895–1905. doi: 10.1097/01.mlg.0000147917.43615.c0. [DOI] [PubMed] [Google Scholar]
- 4.Soler ZM, Sauer D, Mace J, Smith TL. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2010;142:64–71. doi: 10.1016/j.otohns.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi LL, Xiong P, Zhang L, et al. Features of airway remodeling in different types of Chinese chronic rhinosinusitis are associated with inflammation patterns. Allergy. 2013;68:101–109. doi: 10.1111/all.12064. [DOI] [PubMed] [Google Scholar]
- 6.Soler ZM, Sauer DA, Mace J, Smith TL. Relationship between clinical measures and histopathologic findings in chronic rhinosinusitis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2009;141:454–461. doi: 10.1016/j.otohns.2009.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czerny MS, Namin A, Gratton MA, Antisdel JL. Histopathological and clinical analysis of chronic rhinosinusitis by subtype. International forum of allergy & rhinology. 2014;4:463–469. doi: 10.1002/alr.21304. [DOI] [PubMed] [Google Scholar]
- 8.Smith TL, Litvack JR, Hwang PH, et al. Determinants of outcomes of sinus surgery: a multi-institutional prospective cohort study. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2010;142:55–63. doi: 10.1016/j.otohns.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SY, Park JH, Rhee CS, Chung JH, Kim JW. Does eosinophilic inflammation affect the outcome of endoscopic sinus surgery in chronic rhinosinusitis in Koreans? American journal of rhinology & allergy. 2013;27:e166–e169. doi: 10.2500/ajra.2013.27.3959. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharyya N. Radiographic stage fails to predict symptom outcomes after endoscopic sinus surgery for chronic rhinosinusitis. The Laryngoscope. 2006;116:18–22. doi: 10.1097/01.mlg.0000192284.22703.04. [DOI] [PubMed] [Google Scholar]
- 11.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. American journal of respiratory and critical care medicine. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenfeld RM. Clinical practice guideline on adult sinusitis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2007;137:365–377. doi: 10.1016/j.otohns.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clinical otolaryngology : official journal of ENT-UK; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2009;34:447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 14.Benninger MS, Senior BA. The development of the Rhinosinusitis Disability Index. Archives of otolaryngology--head & neck surgery. 1997;123:1175–1179. doi: 10.1001/archotol.1997.01900110025004. [DOI] [PubMed] [Google Scholar]
- 15.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 17.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Medical care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 18.Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT) The Laryngoscope. 1996;106:353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31:183–184. [PubMed] [Google Scholar]
- 20.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1997;117:S35–S40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 21.Schatz M, Hsu JW, Zeiger RS, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. The Journal of allergy and clinical immunology. 2014;133:1549–1556. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Batra PS, Tong L, Citardi MJ. Analysis of comorbidities and objective parameters in refractory chronic rhinosinusitis. The Laryngoscope. 2013;123(Suppl 7):S1–S11. doi: 10.1002/lary.24418. [DOI] [PubMed] [Google Scholar]
- 23.Han JK. Subclassification of chronic rhinosinusitis. The Laryngoscope. 2013;123(Suppl 2):S15–S27. doi: 10.1002/lary.23979. [DOI] [PubMed] [Google Scholar]
- 24.Cao PP, Li HB, Wang BF, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. The Journal of allergy and clinical immunology. 2009;124:478–484. 484, e471–e472. doi: 10.1016/j.jaci.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Carney AS, Tan LW, Adams D, Varelias A, Ooi EH, Wormald PJ. Th2 immunological inflammation in allergic fungal sinusitis, nonallergic eosinophilic fungal sinusitis, and chronic rhinosinusitis. American journal of rhinology. 2006;20:145–149. [PubMed] [Google Scholar]
- 26.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 27.Van Bruaene N, Perez-Novo CA, Basinski TM, et al. T-cell regulation in chronic paranasal sinus disease. The Journal of allergy and clinical immunology. 2008;121:1435–1441. 1441, e1431–e1433. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama T, Asaka D, Yoshikawa M, et al. Identification of chronic rhinosinusitis phenotypes using cluster analysis. American journal of rhinology & allergy. 2012;26:172–176. doi: 10.2500/ajra.2012.26.3749. [DOI] [PubMed] [Google Scholar]


