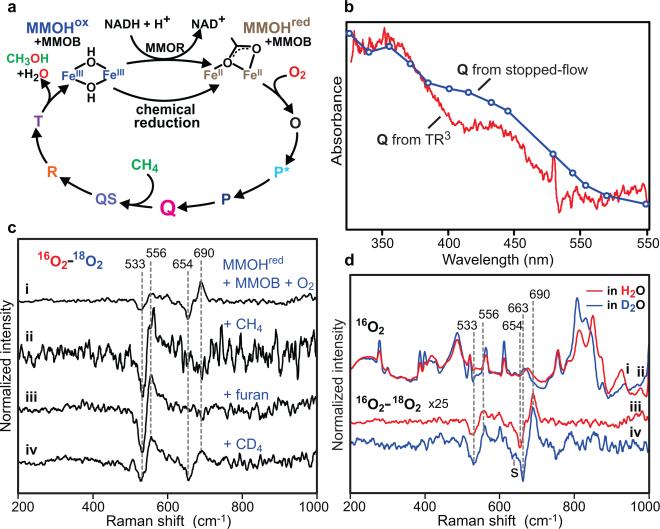
Figure 1. Reaction of sMMO with O2.
a, The catalytic cycle includes stable MMOHox and MMOHred and detectable transient species O, P*, P, Q and T; transient states QS and R were predicted from kinetic, spectroscopic, and chemical studies. b, An in situ transient difference electronic absorption spectrum of the reaction mixture (vs anaerobic MMOHred) in the TR3 instrument at Δt ≈ 3.0 s (red, 1.0 × 0.12 mm probe volume) in comparison with spectrum of Q reconstructed from stopped-flow kinetic traces (markers, blue). c, Transient 16O2 – 18O2 difference resonance Raman spectra of sMMO reveal vibrations of iron-bound oxygen atoms. Measurement conditions: pH 7.0, 4 °C, Δt ≈ 3.0 s. Spectra recorded without the substrate (i) show marked changes when 0.45 mM CH4 (ii), 3.5 mM furan (iii) or 0.45 mM CD4 (iv) is added. d, Solvent vibrations in the absolute resonance Raman spectra are sensitive to H2O (i)/D2O (ii) substitution. 16O2 – 18O2 difference spectra of sMMO recorded in H2O (iii) show little sensitivity to D2O (iv) substitution. The upshift of 18O vibration of Q and the appearance of a low-frequency shoulder (marked with S) in D2O is attributed to Fermi resonance with a protein-derived metal ligand.